International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts
- PMID: 25987659
- PMCID: PMC4504945
- DOI: 10.1182/blood-2015-01-621664
International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts
Abstract
This multicenter, randomized, open-label, phase 3 trial evaluated azacitidine efficacy and safety vs conventional care regimens (CCRs) in 488 patients age ≥65 years with newly diagnosed acute myeloid leukemia (AML) with >30% bone marrow blasts. Before randomization, a CCR (standard induction chemotherapy, low-dose ara-c, or supportive care only) was preselected for each patient. Patients then were assigned 1:1 to azacitidine (n = 241) or CCR (n = 247). Patients assigned to CCR received their preselected treatment. Median overall survival (OS) was increased with azacitidine vs CCR: 10.4 months (95% confidence interval [CI], 8.0-12.7 months) vs 6.5 months (95% CI, 5.0-8.6 months), respectively (hazard ratio [HR] was 0.85; 95% CI, 0.69-1.03; stratified log-rank P = .1009). One-year survival rates with azacitidine and CCR were 46.5% and 34.2%, respectively (difference, 12.3%; 95% CI, 3.5%-21.0%). A prespecified analysis censoring patients who received AML treatment after discontinuing study drug showed median OS with azacitidine vs CCR was 12.1 months (95% CI, 9.2-14.2 months) vs 6.9 months (95% CI, 5.1-9.6 months; HR, 0.76; 95% CI, 0.60-0.96; stratified log-rank P = .0190). Univariate analysis showed favorable trends for azacitidine compared with CCR across all subgroups defined by baseline demographic and disease features. Adverse events were consistent with the well-established safety profile of azacitidine. Azacitidine may be an important treatment option for this difficult-to-treat AML population. This trial was registered at www.clinicaltrials.gov as #NCT01074047.
© 2015 by The American Society of Hematology.
Figures
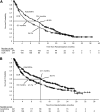
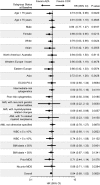
Comment in
-
Azacitidine in AML: a treatment option?Blood. 2015 Jul 16;126(3):283-4. doi: 10.1182/blood-2015-06-648071. Blood. 2015. PMID: 26185114
References
-
- National Cancer Institute. Adult Acute Myeloid Leukemia Treatment (PDQ). Available at: http://www.cancer.gov/cancertopics/pdq/treatment/adultAML/healthprofessi.... Last update: January 5, 2015. Accessed March 18, 2015.
-
- Visser O, Trama A, Maynadié M, et al. RARECARE Working Group. Incidence, survival and prevalence of myeloid malignancies in Europe. Eur J Cancer. 2012;48(17):3257–3266. - PubMed
-
- Dombret H, Raffoux E, Gardin C. Acute myeloid leukemia in the elderly. Semin Oncol. 2008;35(4):430–438. - PubMed
-
- Kantarjian HM, Thomas XG, Dmoszynska A, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012;30(21):2670–2677. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

