Muscular dystrophy in the mdx mouse is a severe myopathy compounded by hypotrophy, hypertrophy and hyperplasia
- PMID: 25987977
- PMCID: PMC4434871
- DOI: 10.1186/s13395-015-0041-y
Muscular dystrophy in the mdx mouse is a severe myopathy compounded by hypotrophy, hypertrophy and hyperplasia
Abstract
Background: Preclinical testing of potential therapies for Duchenne muscular dystrophy (DMD) is conducted predominantly of the mdx mouse. But lack of a detailed quantitative description of the pathology of this animal limits our ability to evaluate the effectiveness of putative therapies or their relevance to DMD.
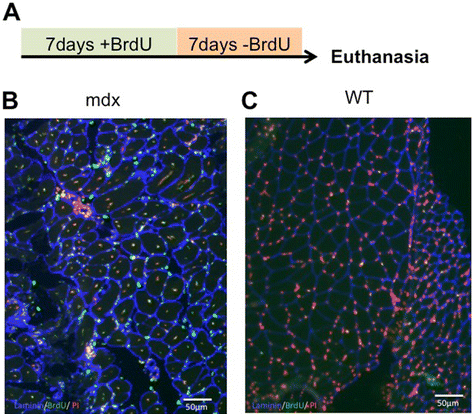
Methods: Accordingly, we have measured the main cellular components of muscle growth and regeneration over the period of postnatal growth and early pathology in mdx and wild-type (WT) mice; phalloidin binding is used as a measure of fibre size, myonuclear counts and BrdU labelling as records of myogenic activity.
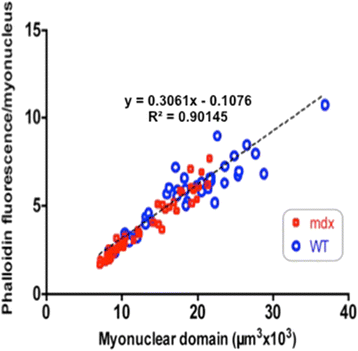
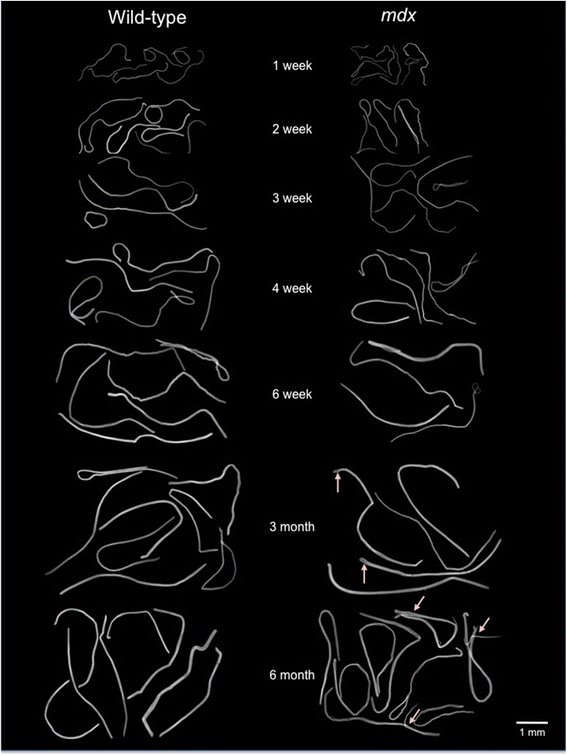
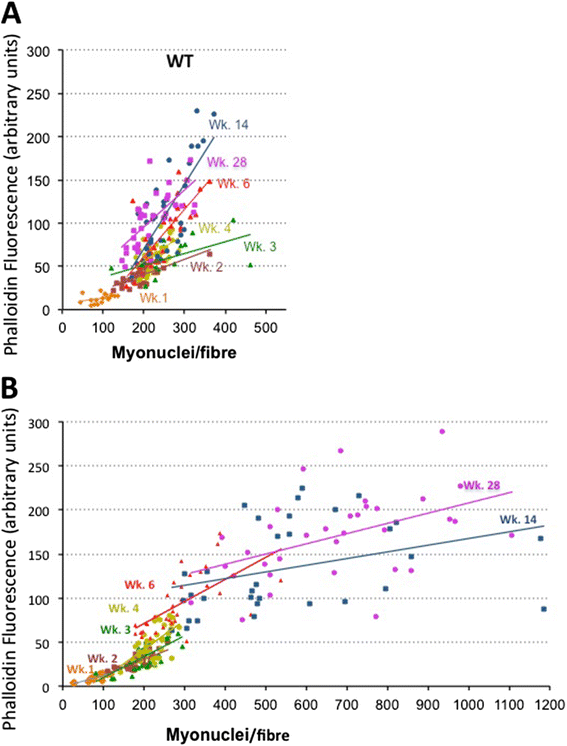
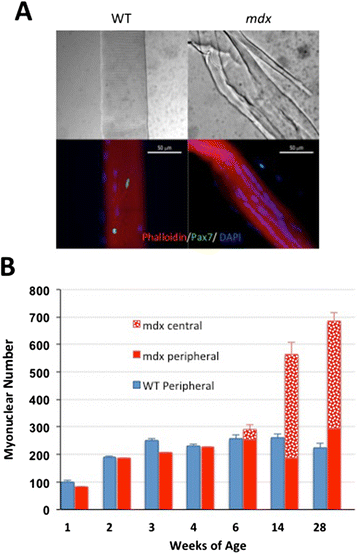
Results: We confirm a two-phase postnatal growth pattern in WT muscle: first, increase in myonuclear number over weeks 1 to 3, then expansion of myonuclear domain. Mdx muscle growth lags behind that of WT prior to overt signs of pathology. Fibres are smaller, with fewer myonuclei and smaller myonuclear domains. Moreover, satellite cells are more readily detached from mdx than WT muscle fibres. At 3 weeks, mdx muscles enter a phase of florid myonecrosis, accompanied by concurrent regeneration of an intensity that results in complete replacement of pre-existing muscle over the succeeding 3 to 4 weeks. Both WT and mdx muscles attain maximum size by 12 to 14 weeks, mdx muscle fibres being up to 50% larger than those of WT as they become increasingly branched. Mdx muscle fibres also become hypernucleated, containing twice as many myonuclei per sarcoplasmic volume, as those of WT, the excess corresponding to the number of centrally placed myonuclei.
Conclusions: The best-known consequence of lack of dystrophin that is common to DMD and the mdx mouse is the conspicuous necrosis and regeneration of muscle fibres. We present protocols for measuring this in terms both of loss of muscle nuclei previously labelled with BrdU and of the intensity of myonuclear labelling with BrdU administered during the regeneration period. Both measurements can be used to assess the efficacy of putative antinecrotic agents. We also show that lack of dystrophin is associated with a number of previously unsuspected abnormalities of muscle fibre structure and function that do not appear to be directly associated with myonecrosis.
Keywords: Hyperplasia; Hypertrophy; Hypotrophy; Muscle regeneration; Muscular dystrophy.
Figures



References
-
- Harris JB, Slater CR. Animal models of muscular dystrophy. Br Med Bull. 1980;36:193–7. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

