Losartan treatment attenuates tumor-induced myocardial dysfunction
- PMID: 25988231
- PMCID: PMC4530048
- DOI: 10.1016/j.yjmcc.2015.05.007
Losartan treatment attenuates tumor-induced myocardial dysfunction
Abstract
Fatigue and muscle wasting are common symptoms experienced by cancer patients. Data from animal models demonstrate that angiotensin is involved in tumor-induced muscle wasting, and that tumor growth can independently affect myocardial function, which could contribute to fatigue in cancer patients. In clinical studies, inhibitors of angiotensin converting enzyme (ACE) can prevent the development of chemotherapy-induced cardiovascular dysfunction, suggesting a mechanistic role for the renin-angiotensin-aldosterone system (RAAS). In the present study, we investigated whether an angiotensin (AT) 1-receptor antagonist could prevent the development of tumor-associated myocardial dysfunction.
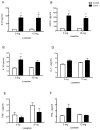
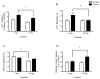
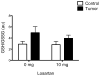
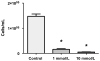
Methods and results: Colon26 adenocarcinoma (c26) cells were implanted into female CD2F1 mice at 8weeks of age. Simultaneously, mice were administered Losartan (10mg/kg) daily via their drinking water. In vivo echocardiography, blood pressure, in vitro cardiomyocyte function, cell proliferation assays, and measures of systemic inflammation and myocardial protein degradation were performed 19days following tumor cell injection. Losartan treatment prevented tumor-induced loss of muscle mass and in vitro c26 cell proliferation, decreased tumor weight, and attenuated myocardial expression of interleukin-6. Furthermore, Losartan treatment mitigated tumor-associated alterations in calcium signaling in cardiomyocytes, which was associated with improved myocyte contraction velocity, systolic function, and blood pressures in the hearts of tumor-bearing mice.
Conclusions: These data suggest that Losartan may mitigate tumor-induced myocardial dysfunction and inflammation.
Keywords: Calcium signaling; Cancer cachexia; Cardiomyocyte; Cardiovascular function; Losartan.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Conflict of interest statement
None declared
Figures



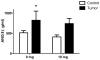
References
-
- Douglas E, McMillan DC. Towards a simple objective framework for the investigation and treatment of cancer cachexia: The Glasgow Prognostic Score. Cancer treatment reviews. 2014;40:685–91. - PubMed
-
- Tian M, Asp ML, Nishijima Y, Belury MA. Evidence for cardiac atrophic remodeling in cancer-induced cachexia in mice. International journal of oncology. 2011;39:1321–6. - PubMed
-
- Zuppinger C, Timolati F, Suter TM. Pathophysiology and diagnosis of cancer drug induced cardiomyopathy. Cardiovascular toxicology. 2007;7:61–6. - PubMed
-
- Tian M, Nishijima Y, Asp ML, Stout MB, Reiser PJ, Belury MA. Cardiac alterations in cancer-induced cachexia in mice. International journal of oncology. 2010;37:347–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

