Low-dose memantine attenuated methadone dose in opioid-dependent patients: a 12-week double-blind randomized controlled trial
- PMID: 25988317
- PMCID: PMC4650802
- DOI: 10.1038/srep10140
Low-dose memantine attenuated methadone dose in opioid-dependent patients: a 12-week double-blind randomized controlled trial
Abstract
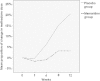
Low-dose memantine might have anti-inflammatory and neurotrophic effects mechanistically remote from an NMDA receptor. We investigated whether add-on memantine reduced cytokine levels and benefitted patients with opioid dependence undergoing methadone maintenance therapy (MMT) in a randomized, double-blind, controlled 12-week study. Patients were randomly assigned to a group: Memantine (5 mg/day) (n = 53) or Placebo (n = 75). The methadone dose required and retention in treatment were monitored. Plasma tumor necrosis factor (TNF)-α, C-reactive protein (CRP), interleukin (IL)-6, IL-8, transforming growth factor (TGF)-β1, and brain-derived neurotrophic factor (BDNF) levels were examined during weeks 0, 1, 4, 8, and 12. General linear mixed models were used to examine therapeutic effect. After 12 weeks, Memantine-group required a somewhat lower methadone dose than did Placebo-group (P = 0.039). They also had significantly lower plasma TNF-α and significantly higher TGF-β1 levels. We provide evidence of the benefit of add-on memantine in opioid dependent patients undergoing MMT.
Figures


References
-
- Mattick R. P., Breen C., Kimber J., Davoli M., Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst. Rev. 2, CD002207 (2014). - PubMed
-
- Thomas P. T., Bhargava H. N., House R. V., Immunomodulatory effects of in vitro exposure to morphine and its metabolites. Pharmacology . 50, 51–62 (1995). - PubMed
-
- Kapasi A. A., Gibbons N., Mattana J., Singhal P. C., Morphine stimulates mesangial cell TNF-alpha and nitrite production. Inflammation . 24, 463–476 (2000). - PubMed
-
- Zubelewicz B., Muc-Wierzgon M., Harbuz M. S., Brodziak A., Central single and chronic administration of morphine stimulates corticosterone and interleukin (IL)-6 in adjuvant-induced arthritis. J. Physiol. Pharmacol. 51, 897–906 (2000). - PubMed
-
- Dyuizen I., Lamash N. E. Histo- and immunocytochemical detection of inducible NOS and TNF-alpha in the locus coeruleus of human opiate addicts. J. Chem. Neuroanat. 37, 65–70 (2009). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

