Ferritin levels in the cerebrospinal fluid predict Alzheimer's disease outcomes and are regulated by APOE
- PMID: 25988319
- PMCID: PMC4479012
- DOI: 10.1038/ncomms7760
Ferritin levels in the cerebrospinal fluid predict Alzheimer's disease outcomes and are regulated by APOE
Abstract
Brain iron elevation is implicated in Alzheimer's disease (AD) pathogenesis, but the impact of iron on disease outcomes has not been previously explored in a longitudinal study. Ferritin is the major iron storage protein of the body; by using cerebrospinal fluid (CSF) levels of ferritin as an index, we explored whether brain iron status impacts longitudinal outcomes in the Alzheimer's Disease Neuroimaging Initiative (ADNI) cohort. We show that baseline CSF ferritin levels were negatively associated with cognitive performance over 7 years in 91 cognitively normal, 144 mild cognitive impairment (MCI) and 67 AD subjects, and predicted MCI conversion to AD. Ferritin was strongly associated with CSF apolipoprotein E levels and was elevated by the Alzheimer's risk allele, APOE-ɛ4. These findings reveal that elevated brain iron adversely impacts on AD progression, and introduce brain iron elevation as a possible mechanism for APOE-ɛ4 being the major genetic risk factor for AD.
Conflict of interest statement
A.I.B. is a shareholder in Prana Biotechnology, Cogstate, Eucalyptus, Mesoblast, Brighton Biotech, LLC, and a payed consultant for Collaborative Medicinal Developments, LLC and Brighton Biotech, LLC. S.A. and N.G.F. declare no competing financial interests.
Figures
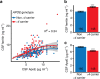
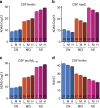
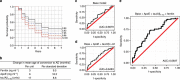
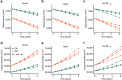
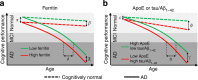
Comment in
-
Alzheimer disease: Iron--the missing link between ApoE and Alzheimer disease?Nat Rev Neurol. 2015 Jul;11(7):369. doi: 10.1038/nrneurol.2015.96. Epub 2015 Jun 9. Nat Rev Neurol. 2015. PMID: 26055466 No abstract available.
References
-
- van Rooden S. et al.. 7T T2-weighted magnetic resonance imaging reveals cortical phase differences between early- and late-onset Alzheimer's disease. Neurobiol. Aging 36, 20–26 (2014). - PubMed
-
- Crapper McLachlan D. R. et al.. Intramuscular desferrioxamine in patients with Alzheimer's disease. Lancet 337, 1304–1308 (1991). - PubMed
-
- Lei P. et al.. Tau deficiency induces parkinsonism with dementia by impairing APP-mediated iron export. Nat. Med. 18, 291–295 (2012). - PubMed
-
- Jellinger K., Paulus W., Grundke-Iqbal I., Riederer P. & Youdim M. B. Brain iron and ferritin in Parkinson's and Alzheimer's diseases. J. Neural Transm. Park. Dis. Dement. Sect. 2, 327–340 (1990). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

