Marine polysaccharides from algae with potential biomedical applications
- PMID: 25988519
- PMCID: PMC4446615
- DOI: 10.3390/md13052967
Marine polysaccharides from algae with potential biomedical applications
Abstract
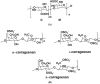
There is a current tendency towards bioactive natural products with applications in various industries, such as pharmaceutical, biomedical, cosmetics and food. This has put some emphasis in research on marine organisms, including macroalgae and microalgae, among others. Polysaccharides with marine origin constitute one type of these biochemical compounds that have already proved to have several important properties, such as anticoagulant and/or antithrombotic, immunomodulatory ability, antitumor and cancer preventive, antilipidaemic and hypoglycaemic, antibiotics and anti-inflammatory and antioxidant, making them promising bioactive products and biomaterials with a wide range of applications. Their properties are mainly due to their structure and physicochemical characteristics, which depend on the organism they are produced by. In the biomedical field, the polysaccharides from algae can be used in controlled drug delivery, wound management, and regenerative medicine. This review will focus on the biomedical applications of marine polysaccharides from algae.
Keywords: algae; bioactive; biomedical; drug delivery; pharmaceuticals; polysaccharides; regenerative medicine; therapeutics; wound management.
Figures



References
-
- Costa L.S., Fidelis G.P., Cordeiro S.L., Oliveira R.M., Sabry D.A., Câmara R.B.G., Nobre L.T.D.B., Costa M.S.S.P., Almeida-Lima J., Farias E.H.C., et al. Biological activities of sulfated polysaccharides from tropical seaweeds. Biomed. Pharmacother. 2010;64:21–28. doi: 10.1016/j.biopha.2009.03.005. - DOI - PubMed
-
- Jiménez-Escrig A., Gómez-Ordóñez E., Rupérez P. Seaweed as a source of novel nutraceuticals: Sulfated polysaccharides and peptides. Adv. Food Nutr. Res. 2011;64:325–337. - PubMed
-
- Kraan S. Carbohydrates-Comprehensive Studies on Glycobiology and Glycotechnology. InTech; Rijeka, Croatia: 2012. Algal polysaccharides, novel applications and outlook; pp. 489–524. Chapter 22.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

