RSK3: A regulator of pathological cardiac remodeling
- PMID: 25988524
- PMCID: PMC4449288
- DOI: 10.1002/iub.1383
RSK3: A regulator of pathological cardiac remodeling
Abstract
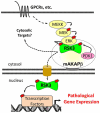
The family of p90 ribosomal S6 kinases (RSKs) are pleiotropic effectors for extracellular signal-regulated kinase signaling pathways. Recently, RSK3 was shown to be important for pathological remodeling of the heart. Although cardiac myocyte hypertrophy can be compensatory for increased wall stress, in chronic heart diseases, this nonmitotic cell growth is usually associated with interstitial fibrosis, increased cell death, and decreased cardiac function. Although RSK3 is less abundant in the cardiac myocyte than other RSK family members, RSK3 appears to serve a unique role in cardiac myocyte stress responses. A potential mechanism conferring the unique function of RSK3 in the heart is anchoring by the scaffold protein muscle A-kinase anchoring protein β (mAKAPβ). Recent findings suggest that RSK3 should be considered as a therapeutic target for the prevention of heart failure, a clinical syndrome of major public health significance.
Keywords: Keywords ribosomal S6 kinase; heart; hypertrophy; mAKAP; remodeling; scaffold; signal transduction.
© 2015 International Union of Biochemistry and Molecular Biology.
Figures


References
-
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB, American Heart Association Statistics C. Stroke Statistics S Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. - PMC - PubMed
-
- Braunwald E. Heart failure. JACC Heart failure. 2013;1:1–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

