Differential dependence on nuclear factor-κB-inducing kinase among natural killer T-cell subsets in their development
- PMID: 25988531
- PMCID: PMC4552504
- DOI: 10.1111/imm.12484
Differential dependence on nuclear factor-κB-inducing kinase among natural killer T-cell subsets in their development
Abstract
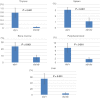
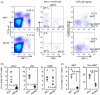
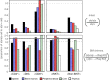
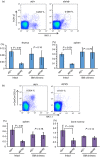
Natural killer T cells (NKT cells) are comprised of several subsets. However, the possible differences in their developmental mechanisms have not been fully investigated. To evaluate the dependence of some NKT subpopulations on nuclear factor-κB-inducing kinase (NIK) for their generation, we analysed the differentiation of NKT cells, dividing them into subsets in various tissues of alymphoplasia (aly/aly), a mutant mouse strain that lacks functional NIK. The results indicated that the efficient differentiation of both invariant NKT (iNKT) and non-iNKT cells relied on NIK expression in non-haematopoietic cells; however, the dependence of non-iNKT cells was lower than that of iNKT cells. Especially, the differentiation of CD8(+) non-iNKT cells was markedly resistant to the aly mutation. The proportion of two other NKT cell subsets, NK1.1(+) γδ T cells and NK1.1(-) iNKT cells, was also significantly reduced in aly/aly mice, and this defect in their development was reversed in wild-type host mice given aly/aly bone marrow cells. In exerting effector functions, NIK in NKT-αβ cells appeared dispensable, as NIK-deficient NKT-αβ cells could secrete interleukin-4 or interferon-γ and exhibit cytolytic activity at a level comparable to that of aly/+ NKT-αβ cells. Collectively, these results imply that the NIK in thymic stroma may be critically involved in the differentiation of most NKT cell subsets (although the level of NIK dependence may vary among the subsets), and also that NIK in NKT-αβ cells may be dispensable for their effector function.
Keywords: differentiation; natural killer T cells; nuclear factor-κB-inducing kinase.
© 2015 John Wiley & Sons Ltd.
Figures


Similar articles
-
NIK-dependent RelB activation defines a unique signaling pathway for the development of V alpha 14i NKT cells.J Exp Med. 2003 Jun 16;197(12):1623-33. doi: 10.1084/jem.20030141. J Exp Med. 2003. PMID: 12810685 Free PMC article.
-
Significant involvement of nuclear factor-κB-inducing kinase in proper differentiation of αβ and γδ T cells.Immunology. 2014 Feb;141(2):222-32. doi: 10.1111/imm.12186. Immunology. 2014. PMID: 24117043 Free PMC article.
-
Mammalian target of rapamycin complex 1 orchestrates invariant NKT cell differentiation and effector function.J Immunol. 2014 Aug 15;193(4):1759-65. doi: 10.4049/jimmunol.1400769. Epub 2014 Jul 11. J Immunol. 2014. PMID: 25015820
-
Tec family kinases: Itk signaling and the development of NKT αβ and γδ T cells.FEBS J. 2011 Jun;278(12):1970-9. doi: 10.1111/j.1742-4658.2011.08074.x. Epub 2011 Mar 29. FEBS J. 2011. PMID: 21362141 Review.
-
Thymus medulla fosters generation of natural Treg cells, invariant γδ T cells, and invariant NKT cells: what we learn from intrathymic migration.Eur J Immunol. 2015 Mar;45(3):652-60. doi: 10.1002/eji.201445108. Epub 2015 Feb 13. Eur J Immunol. 2015. PMID: 25615828 Free PMC article. Review.
Cited by
-
NF-κB-inducing kinase contributes to normal development of cortical thymic epithelial cells: its possible role in shaping a proper T-cell repertoire.Immunology. 2020 Jun;160(2):198-208. doi: 10.1111/imm.13186. Epub 2020 Apr 13. Immunology. 2020. PMID: 32145062 Free PMC article.
-
Pathology and molecular mechanisms of Schistosoma japonicum-associated liver fibrosis.Front Cell Infect Microbiol. 2022 Oct 28;12:1035765. doi: 10.3389/fcimb.2022.1035765. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36389166 Free PMC article. Review.
-
30 Years of NF-κB: A Blossoming of Relevance to Human Pathobiology.Cell. 2017 Jan 12;168(1-2):37-57. doi: 10.1016/j.cell.2016.12.012. Epub 2017 Jan 12. Cell. 2017. PMID: 28086098 Free PMC article. Review.
References
-
- Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

