Pelitinib (EKB-569) targets the up-regulation of ABCB1 and ABCG2 induced by hyperthermia to eradicate lung cancer
- PMID: 25988710
- PMCID: PMC4543615
- DOI: 10.1111/bph.13189
Pelitinib (EKB-569) targets the up-regulation of ABCB1 and ABCG2 induced by hyperthermia to eradicate lung cancer
Abstract
Background and purpose: Pelitinib is a potent irreversible EGFR TK inhibitor currently in clinical trials for the treatment of lung cancer. Hyperthermia has been applied concomitantly with chemotherapy and radiotherapy to enhance treatment outcome. In this study, we investigated the ability of the combination of pelitinib with other conventional anticancer drugs to specifically target cancer cells with up-regulated efflux transporters ABCB1/ABCG2 after hyperthermia as a novel way to eradicate the cancer stem-like cells responsible for cancer recurrence.
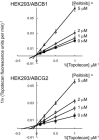
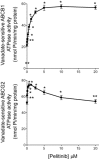
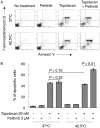
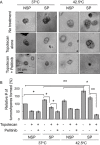
Experimental approach: Alterations in intracellular topotecan accumulation, the efflux of fluorescent probe substrates, expression and ATPase activity of ABCB1/ABCG2 and tumoursphere formation capacity of side population (SP) cells sorted after hyperthermia were examined to elucidate the mechanism of pelitinib-induced chemosensitization.
Key results: While pelitinib did not modulate ABCB1/ABCG2 expressions, the combination of pelitinib with transporter substrate anticancer drugs induced more marked apoptosis, specifically in cells exposed to hyperthermia. The flow cytometric assay showed that both ABCB1- and ABCG2-mediated drug effluxes were significantly inhibited by pelitinib in a concentration-dependent manner. The inhibition kinetics suggested that pelitinib is a competitive inhibitor of ABCB1/ABCG2, which is consistent with its ability to stimulate their ATPase activity. SP cells sorted after hyperthermia were found to be more resistant to anticancer drugs, presumably due to the up-regulation of ABCB1 and ABCG2. Importantly, pelitinib specifically enhanced the chemosensitivity but reduced the tumoursphere formation capacity of these SP cells.
Conclusions and implications: This study demonstrated a novel approach, exploiting drug resistance, to selectively kill cancer stem-like cells after hyperthermia.
© 2015 The British Pharmacological Society.
Figures





References
-
- Ambudkar SV. Drug-stimulatable ATPase activity in crude membranes of human MDR1-transfected mammalian cells. Methods Enzymol. 1998;292:504–514. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

