Intrinsic host restrictions to HIV-1 and mechanisms of viral escape
- PMID: 25988886
- PMCID: PMC6908429
- DOI: 10.1038/ni.3156
Intrinsic host restrictions to HIV-1 and mechanisms of viral escape
Abstract
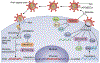
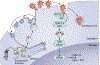
To replicate in their hosts, viruses have to navigate the complexities of the mammalian cell, co-opting mechanisms of cellular physiology while defeating restriction factors that are dedicated to halting their progression. Primate lentiviruses devote a relatively large portion of their coding capacity to counteracting restriction factors by encoding accessory proteins dedicated to neutralizing the antiviral function of these intracellular inhibitors. Research into the roles of the accessory proteins has revealed the existence of previously undetected intrinsic defenses, provided insight into the evolution of primate lentiviruses as they adapt to new species and uncovered new targets for the development of therapeutics. This Review discusses the biology of the restriction factors APOBEC3, SAMHD1 and tetherin and the viral accessory proteins that counteract them.
Conflict of interest statement
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Figures


References
-
- Strebel K et al. The HIV ‘A’ (sor) gene product is essential for virus infectivity. Nature 328, 728–730 (1987). - PubMed
-
- Cullen BR HIV-1 Vif: counteracting innate antiretroviral defenses. Mol. Ther 8, 525–527 (2003). - PubMed
-
- Simon JH, Gaddis NC, Fouchier RA & Malim MH Evidence for a newly discovered cellular anti-HIV-1 phenotype. Nat. Med 4, 1397–1400 (1998). - PubMed
-
- Sheehy AM, Gaddis N, Choi J & Malim M Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature 418, 646–650 (2002). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

