The Subjective Experience of Pain: An FMRI Study of Percept-Related Models and Functional Connectivity
- PMID: 25989475
- PMCID: PMC4653099
- DOI: 10.1111/pme.12785
The Subjective Experience of Pain: An FMRI Study of Percept-Related Models and Functional Connectivity
Abstract
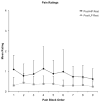
Objective: Previous work suggests that the perception of pain is subjective and dependent on individual differences in physiological, emotional, and cognitive states. Functional magnetic resonance imaging (FMRI) studies have used both stimulus-related (nociceptive properties) and percept-related (subjective experience of pain) models to identify the brain networks associated with pain. Our objective was to identify the network involved in processing subjective pain during cold stimuli.
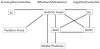
Methods: The current FMRI study directly contrasted a stimulus-related model with a percept-related model during blocks of cold pain stimuli in healthy adults. Specifically, neuronal activation was modelled as a function of changes in stimulus intensity vs as a function of increasing/decreasing levels of subjective pain corresponding to changes in pain ratings. In addition, functional connectivity analyses were conducted to examine intrinsic correlations between three proposed subnetworks (sensory/discriminative, affective/motivational, and cognitive/evaluative) involved in pain processing.
Results: The percept-related model captured more extensive activation than the stimulus-related model and demonstrated an association between higher subjective pain and activation in expected cortical (dorsolateral prefrontal cortex, ventrolateral prefrontal cortex, insula, dorsal anterior cingulate cortex [dACC] extending into pre-supplementary motor area) and subcortical (thalamus, striatum) areas. Moreover, connectivity results supported the posited roles of dACC and insula as key relay sites during neural processing of subjective pain. In particular, anterior insula appeared to link sensory/discriminative regions with regions in the other subnetworks, and dACC appeared to serve as a hub for affective/motivational, cognitive/evaluative, and motor subnetworks.
Conclusions: Using a percept-related model, brain regions involved in the processing of subjective pain during the application of cold stimuli were identified. Connectivity analyses identified linkages between key subnetworks involved in processing subjective pain.
Keywords: Connectivity; Functional Magnetic Resonance Imaging; Pain; Percept-Related; Ratings.
Wiley Periodicals, Inc.
Conflict of interest statement
The authors have no conflicts of interest to report.
Figures




References
-
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–84. - PubMed
-
- Davis KD, Moayedi M. Central mechanisms of pain revealed through functional and structural MRI. J Neuroimmune Pharmacol. 2012;8:518–34. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

