Rapid COJEC versus standard induction therapies for high-risk neuroblastoma
- PMID: 25989478
- PMCID: PMC10501324
- DOI: 10.1002/14651858.CD010774.pub2
Rapid COJEC versus standard induction therapies for high-risk neuroblastoma
Abstract
Background: Neuroblastoma is a rare malignant disease and mainly affects infants and very young children. The tumors mainly develop in the adrenal medullary tissue and an abdominal mass is the most common presentation. The high-risk group is characterized by metastasis and other characteristics that increase the risk for an adverse outcome. In the rapid COJEC induction schedule, higher single doses of selected drugs than standard induction schedules are administered over a substantially shorter treatment period, with shorter intervals between cycles. Shorter intervals and higher doses increase the dose intensity of chemotherapy and might improve survival.
Objectives: The aim of this study was to evaluate the efficacy and adverse events of the rapid COJEC induction schedule as compared to standard induction schedules in patients with high-risk neuroblastoma (as defined by the International Neuroblastoma Risk Group (INRG) classification system). Outcomes of interest were complete response, early toxicity and treatment-related mortality as primary endpoints and overall survival, progression- and event-free survival, late non-hematological toxicity, and health-related quality of life as secondary endpoints.
Search methods: We searched the electronic databases CENTRAL (2014, Issue 11), MEDLINE (PubMed), and EMBASE (Ovid) for articles from inception to 11 November 2014. Further searches included trial registries, conference proceedings, and reference lists of recent reviews and relevant articles. We did not apply limits on publication year or languages.
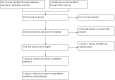
Selection criteria: Randomized controlled trials evaluating the rapid COJEC induction schedule for high-risk neuroblastoma patients compared to standard induction schedules.
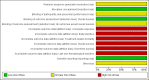
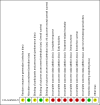
Data collection and analysis: Two review authors performed study selection, abstracted data on study and patient characteristics, and assessed risk of bias independently. We resolved differences by discussion or by appeal to a third review author. We performed analyses according to the guidelines of the Cochrane Handbook for Systematic Reviews of Interventions. We used the five GRADE considerations, study limitations, consistency of effect, imprecision, indirectness, and publication bias, to judge the quality of the evidence. We downgraded for risk of bias and imprecision
Main results: We identified one randomized controlled trial (CCLG-ENSG-5) that included 262 patients with high-risk neuroblastoma who were randomized to receive either rapid COJEC (N = 130) or standard OPEC/COJEC (N = 132) induction chemotherapy. We graded the evidence as low quality; we downgraded for risk of bias and imprecision.There was no clear evidence of a difference between the treatment groups in complete response (risk ratio (RR) 0.99, 95% confidence interval (CI) 0.71 to 1.38), treatment-related mortality (RR 1.21, 95% CI 0.33 to 4.39), overall survival (hazard ratio (HR) 0.83, 95% CI 0.63 to 1.10), and event-free survival (HR 0.86, 95% CI 0.65 to 1.13). We calculated the HRs using the complete follow-up period of the trial.Febrile neutropenia (two or more episodes), proven fungal infections, septicemia (one or more episodes), gastrointestinal toxicity (grade 3 or 4), renal toxicity (glomerular filtration rate < 80 ml/min per body surface area of 1.73 m(2)), neurological toxicity (grade 3 or 4), and ototoxicity (Brock grade 2 to 4) were addressed as early toxicities (during pre-operative chemotherapy). For febrile neutropenia, septicemia, and renal toxicity, a statistically significant difference in favor of the standard treatment arm was identified; for all other early toxicities no clear evidence of a difference between treatment groups was identified. With regard to late non-hematological toxicities (median follow-up 12.7 years; range 6.9 to 16.5 years), the study provided data on any complication, renal toxicity (glomerular filtration rate < 80 ml/min per body surface area of 1.73m(2)), ototoxicity (Brock grade 1 to 4), endocrine complications, neurocognitive complications (i.e. behavioral, speech, or learning difficulties), and second malignancies. For endocrine complications and neurocognitive complications, a statistically significant difference in favor of the rapid COJEC arm was found; for all other late non-hematological toxicities no clear evidence of a difference between treatment groups was identified.Data on progression-free survival and health-related quality of life were not reported.
Authors' conclusions: We identified one randomized controlled trial that evaluated rapid COJEC versus standard induction therapy in patients with high-risk neuroblastoma. No clear evidence of a difference in complete response, treatment-related mortality, overall survival, and event-free survival between the treatment alternatives was found. This could be the result of low power or too short a follow-up period. Results of both early and late toxicities were ambiguous. Information on progression-free survival and health-related quality of life were not available. This trial was performed in the 1990s. Since then, many changes in, for example, treatment and risk classification have occurred. Therefore, based on the currently available evidence, we are uncertain about the effects of rapid COJEC and standard induction therapy in patients with high-risk neuroblastoma. More research is needed for a definitive conclusion.
Conflict of interest statement
FP declares no conflicts of interest.
DAT declares no conflicts of interest.
ECVD declares no conflicts of interest.
FP declares no conflicts of interest.
Figures
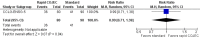
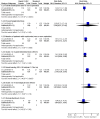
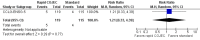

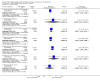




Update of
- doi: 10.1002/14651858.CD010774
Similar articles
-
Retinoic acid post consolidation therapy for high-risk neuroblastoma patients treated with autologous hematopoietic stem cell transplantation.Cochrane Database Syst Rev. 2015 Jan 29;1:CD010685. doi: 10.1002/14651858.CD010685.pub2. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2017 Aug 25;8:CD010685. doi: 10.1002/14651858.CD010685.pub3. PMID: 25634649 Updated.
-
Retinoic acid postconsolidation therapy for high-risk neuroblastoma patients treated with autologous haematopoietic stem cell transplantation.Cochrane Database Syst Rev. 2017 Aug 25;8(8):CD010685. doi: 10.1002/14651858.CD010685.pub3. Cochrane Database Syst Rev. 2017. PMID: 28840597 Free PMC article.
-
Comparison of first-line chemotherapy including escalated BEACOPP versus chemotherapy including ABVD for people with early unfavourable or advanced stage Hodgkin lymphoma.Cochrane Database Syst Rev. 2017 May 25;5(5):CD007941. doi: 10.1002/14651858.CD007941.pub3. Cochrane Database Syst Rev. 2017. PMID: 28541603 Free PMC article.
-
High-dose chemotherapy and autologous haematopoietic stem cell rescue for children with high-risk neuroblastoma.Cochrane Database Syst Rev. 2015 Oct 5;2015(10):CD006301. doi: 10.1002/14651858.CD006301.pub4. Cochrane Database Syst Rev. 2015. PMID: 26436598 Free PMC article.
-
Chemotherapy for children with medulloblastoma.Cochrane Database Syst Rev. 2015 Jan 1;1(1):CD006678. doi: 10.1002/14651858.CD006678.pub2. Cochrane Database Syst Rev. 2015. PMID: 25879092 Free PMC article.
Cited by
-
MYCN Impact on High-Risk Neuroblastoma: From Diagnosis and Prognosis to Targeted Treatment.Cancers (Basel). 2022 Sep 12;14(18):4421. doi: 10.3390/cancers14184421. Cancers (Basel). 2022. PMID: 36139583 Free PMC article. Review.
-
Anti-GD2 antibody-containing immunotherapy postconsolidation therapy for people with high-risk neuroblastoma treated with autologous haematopoietic stem cell transplantation.Cochrane Database Syst Rev. 2019 Apr 24;4(4):CD012442. doi: 10.1002/14651858.CD012442.pub2. Cochrane Database Syst Rev. 2019. PMID: 31016728 Free PMC article.
-
Targeting the p53-MDM2 pathway for neuroblastoma therapy: Rays of hope.Cancer Lett. 2021 Jan 1;496:16-29. doi: 10.1016/j.canlet.2020.09.023. Epub 2020 Sep 29. Cancer Lett. 2021. PMID: 33007410 Free PMC article. Review.
-
The efficacy and safety of Iodine-131-metaiodobenzylguanidine therapy in patients with neuroblastoma: a meta-analysis.BMC Cancer. 2022 Feb 28;22(1):216. doi: 10.1186/s12885-022-09329-2. BMC Cancer. 2022. PMID: 35227236 Free PMC article.
-
MTHFR and VDR Polymorphisms Improve the Prognostic Value of MYCN Status on Overall Survival in Neuroblastoma Patients.Int J Mol Sci. 2020 Apr 14;21(8):2714. doi: 10.3390/ijms21082714. Int J Mol Sci. 2020. PMID: 32295184 Free PMC article.
References
References to studies included in this review
CCLG‐ENSG‐5 {published and unpublished data}
-
- Moreno L, Vaidya S, Pinkerton R, Lewis IJ, Imeson J, Ellershaw C, et al. Abstract PL36. Long‐term toxicity in survivors of ENSG5 trial for children with high risk neuroblastoma. The Advances in Neuroblastoma Research 2010 conference, 21 to 24 June 2010 in Stockholm, Sweden. Stockholm: Advances in Neuroblastoma Research Association (ANRA), 2010:105.
-
- Moreno L, Vaidya S, Pinkerton R, Lewis IJ, Imeson J, Ellershaw C, et al. Abstract SEL10. Persistence of disease in long‐term survivors of high‐risk neuroblastoma. Analysis of ENSG5 cooperative trial. The Advances in Neuroblastoma Research 2010 conference, 21 to 24 June 2010 in Stockholm, Sweden. Stockholm: Advances in Neuroblastoma Research Association (ANRA), 2010:136.
-
- Moreno L, Vaidya SJ, Pinkerton CR, Lewis IJ, Imeson J, Machin D, et al. Long‐term follow‐up of children with high‐risk neuroblastoma: the ENSG5 trial experience. Pediatric Blood and Cancer 2013;60(7):1135‐40. - PubMed
-
- Pearson AD, Pinkerton CR, Lewis IJ, Imeson J, Ellershaw C, Machin D. High‐dose rapid and standard induction chemotherapy for patients aged over 1 year with stage 4 neuroblastoma: a randomised trial. Lancet Oncology 2008;9(3):247‐56. - PubMed
References to studies excluded from this review
Bomken 2011 {published data only}
-
- Bomken S, Davies B, Chong L, Cole M, Wood KM, McDermott M, et al. Percentage tumor necrosis following chemotherapy in neuroblastoma correlates with MYCN status but not survival. Pediatric Hematology and Oncology 2011;28(2):106‐14. - PubMed
Castel 2004 {published data only}
-
- Castel V, Canete A. A comparison of current neuroblastoma chemotherapeutics. Expert Opinion on Pharmacotherapy 2004;5(1):71‐80. - PubMed
De Bernardi 2003 {published data only}
-
- Bernardi B, Nicolas B, Boni L, Indolfi P, Carli M, Cordero di Montezemolo L, et al. Disseminated neuroblastoma in children older than one year at diagnosis: comparable results with three consecutive high‐dose protocols adopted by the Italian Co‐Operative Group for Neuroblastoma. Journal of Clinical Oncology 2003;21(8):1592‐601. - PubMed
Habib 2012 {published data only}
Hero 1997 {published data only}
-
- Hero B, Kremens B, Klingebiel T, Bender‐Gotze C, Burdach S, Schrappe M, et al. Does megatherapy contribute to survival in metastatic neuroblastoma? A retrospective analysis. German Cooperative Neuroblastoma Study Group. Klinische Pädiatrie 1997;209(4):196‐200. - PubMed
Kogner 2004 {published data only}
-
- Kogner P, Borgström P, Karpe B, Lundell G, Hjelm S, Winiarski J, et al. Children with high‐risk neuroblastoma may be long‐term survivors after application of intensified multimodal therapy. SIOP 2004 meeting abstracts: P.C.005. Pediatric Blood and Cancer 2004;43(S6):400.
Ladenstein 2004 {published data only}
-
- Ladenstein R, Pötschger U, Modritz D, Schreier G, Castel V, Michon J, et al. The SIOPEN‐R‐NET project: Building a European network for neuroblastoma treatment (HR‐NBL‐1/ESIOP trial) and research. SIOP 2004 meeting abstract: P.C.014. Pediatric Blood and Cancer 2004;43(S6):402.
Ladenstein 2010 {published data only}
-
- Ladenstein R, Valteau CD, Brock P, Yaniv I, Castel V, Laureys G, et al. Randomized trial of prophylactic granulocyte colony‐stimulating factor during rapid COJEC induction in pediatric patients with high‐risk neuroblastoma: the European HR‐NBL1/SIOPEN study. Journal of Clinical Oncology 2010;28(21):3516‐24. - PubMed
Ladenstein 2011 {published data only}
-
- Ladenstein R, Poetschger U, Boubaker A, Bar‐Sever Z, Drake B, Staudenherz A, et al. The prognostic value of semi‐quantitative I‐123 MIBG scintigraphy at diagnosis in high risk neuroblastoma: Validation of the siopen score method. SIOP 2011 meeting abstract: O102. Pediatric Blood and Cancer 2011;57(5):732‐3.
Ladenstein 2011a {published data only}
-
- Ladenstein R. Recent advancements for high risk neuroblastoma (HRN) in Europe through the siop Europe neuroblastoma group (SIOPEN). European Multidisciplinary Cancer Congress 2011 meeting abstract: 102. European Journal of Cancer 2011;47 Suppl 1:S26.
Ladenstein 2011b {published data only}
-
- Ladenstein R, Poetschger U, Luksch R, Brock P, Castel V, Yaniv I, et al. Busulphan‐melphalan is the superior myeloablative therapy (MAT) for high risk neuroblastoma: Results from the HR‐NBL1/siopen trial. SIOP 2011 meeting abstract: O103. Pediatric Blood and Cancer 2011;57(5):733.
Ladenstein 2012 {published data only}
-
- Ladenstein R, Poetschger U, Luksch R, Brock P, Castel V, Yaniv I, et al. Major results from the HR‐NBl1/siopen trial for high risk neuroblastoma. SIOP 2012 meeting abstract: O069. Pediatric Blood and Cancer 2012;59(6):988‐9.
Ladenstein 2012a {published data only}
-
- Ladenstein R, Potschger U, Lewington V, Bar‐Sever Z, Lambert B, Oudoux A, et al. Update on the prognostic value of the SIOPEN skeletal scoring method in the high‐risk stage 4 neuroblastoma by semi‐quantitative I‐123‐mIBG scintigraphy. OGKJ 2012 meeting abstract. Monatsschrift fur Kinderheilkunde. 2012:271.
Lewington 2011 {published data only}
-
- Lewington V, Poetschger U, Boubaker A, Bar‐Sever Z, Drake B, Staudenherz A, et al. The prognostic value of semi‐quantitative 123I mIBG scintigraphy at diagnosis in high‐risk neuroblastoma: Validation of the SIOPEN score method [9511]. ASCO 2011 Annual Meeting; Journal of Clinical Oncology. 2011; Vol. 29, supplement:http://meetinglibrary.asco.org/content/83036‐102.
Mastrangelo 2011 {published data only}
-
- Mastrangelo S, Rufini V, Ruggiero A, Giannatale A, Riccardi R. Treatment of advanced neuroblastoma in children over 1 year of age: The critical role of 131I‐metaiodobenzylguanidine combined with chemotherapy in a rapid induction regimen. Pediatric Blood and Cancer 2011;56:1032‐40. - PubMed
Nitschke 1980 {published data only}
-
- Nitschke R, Cangir A, Crist W, Berry DH. Intensive chemotherapy for metastatic neuroblastoma: a Southwest Oncology Group study. Medical and Pediatric Oncology 1980;8(3):281‐8. - PubMed
Pinkerton 2000 {published data only}
-
- Pinkerton CR, Blanc Vincent MP, Bergeron C, Fervers B, Philip T. Induction chemotherapy in metastatic neuroblastoma‐‐does dose influence response? A critical review of published data standards, options and recommendations (SOR) project of the National Federation of French Cancer Centres (FNCLCC). European Journal of Cancer 2000;36(14):1808‐15. - PubMed
Sawaguchi 1990 {published data only}
-
- Sawaguchi S, Kaneko M, Uchino J, Takeda T, Iwafuchi M, Matsuyama S, et al. Treatment of advanced neuroblastoma with emphasis on intensive induction chemotherapy. A report from the Study Group of Japan. Cancer 1990;66:1879‐87. - PubMed
Schrey 2013 {published data only}
-
- Schrey D, Vaidya S, Levine D, Moreno L, Pearson A. Inadequate response to induction chemotherapy in children with high‐risk neuroblastoma: An institutional analysis [P‐0244]. 45th Congress of The International Society of Paediatric Oncology (SIOP), Hong Kong; Pediatric Blood and Cancer 2013; Vol. 60, issue S3:111.
Shafford 1984 {published data only}
-
- Shafford EA, Rogers DW, Pritchard J. Advanced neuroblastoma: improved response rate using a multiagent regimen (OPEC) including sequential cisplatin and VM‐26. Journal of Clinical Oncology 1984;2(7):742‐7. - PubMed
Tweddle 2001 {published data only}
-
- Tweddle DA, Pinkerton CR, Lewis IJ, Ellershaw C, Cole M, Pearson AD. OPEC/OJEC for stage 4 neuroblastoma in children over 1 year of age. Medical and Pediatric Oncology 2001;36(1):239‐42. - PubMed
Valteau‐Couanet 2014 {published data only}
-
- Valteau‐Couanet D, Deley MC, Bergeron C, Ducassou S, Michon J, Rubie H, et al. Long‐term results of the combination of the N7 induction chemotherapy and the busulfan‐melphalan high dose chemotherapy. Pediatric Blood and Cancer 2014;61(6):977‐81. - PubMed
Viprey 2014 {published data only}
-
- Viprey VF, Gregory WM, Corrias MV, Tchirkov A, Swerts K, Vicha A, et al. Neuroblastoma mRNAs predict outcome in children with stage 4 neuroblastoma: a European HR‐NBL1/SIOPEN study. Journal of Clinical Oncology 2014;32(10):1074‐83. - PubMed
References to ongoing studies
HR‐NBL1/SIOPEN {published data only}
-
- Ladenstein A. High Risk Neuroblastoma Study 1 (1.5) of SIOP‐Europe (SIOPEN). ClinicalTrials.gov; ID: NCT01704716; URL: http://clinicaltrials.gov/show/NCT01704716 (accessed 2 April 2014).
Additional references
Berthold 2005
-
- Berthold F, Boos J, Burdach S, Erttmann R, Henze G, Hermann J, et al. Myeloablative megatherapy with autologous stem‐cell rescue versus oral maintenance chemotherapy as consolidation treatment in patients with high‐risk neuroblastoma: a randomised controlled trial. Lancet Oncology 2005;6(9):649‐58. - PubMed
Brisse 2011
-
- Brisse HJ, McCarville MB, Granata C, Krug KB, Wootton‐Gorges SL, Kanegawa K, et al. Guidelines for imaging and staging of neuroblastic tumors: consensus report from the International Neuroblastoma Risk Group Project. Radiology 2011;261(1):243‐57. - PubMed
Brock 1991
-
- Brock PR, Bellman SC, Yeomans EC, Pinkerton CR, Pritchard J. Cisplatin ototoxicity in children: A practical grading system. Medical and Pediatric Oncology 1991;19(4):295–300. - PubMed
Brodeur 1993
-
- Brodeur GM, Pritchard J, Berthold F, Carlsen NL, Castel V, Castelberry RP, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. Journal of Clinical Oncology 1993;11(8):1466‐77. - PubMed
Cheung 2001
-
- Cheung NK, Kushner BH, LaQuaglia M, Kramer K, Gollamudi S, Heller G, et al. N7: a novel multi‐modality therapy of high risk neuroblastoma (NB) in children diagnosed over 1 year of age. Medical and Pediatric Oncology 2001;36(1):227‐30. - PubMed
ClinicalTrials.gov 2014
-
- NIH National Institutes of Health. ClinicalTrials.gov. http://clinicaltrials.gov/.
Cohn 2009
Cole 2012
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. - PubMed
EndNote 2014 [Computer program]
-
- Thomson Reuters. EndNote. Version X7. New York City: Thomson Reuters, 2014.
Esiashvili 2007
-
- Esiashvili N, Goodman M, Ward K, Marcus RB Jr, Johnstone PA. Neuroblastoma in adults: incidence and survival analysis based on SEER data. Pediatric Blood and Cancer 2007;49(1):41‐6. - PubMed
GARD 2013
-
- Genetic and Rare Diseases Information Center (GARD), The Office of Rare Diseases Research (ORDR). Neuroblastoma. http://rarediseases.info.nih.gov/GARD/Condition/7185/Neuroblastoma.aspx (accessed 8 July 2013).
Goodman 2012
-
- Goodman MT, Gurney JG, Smith MA, Olshan AF. SEER Pediatric Monograph. Sympathetic Nervous System Tumors. http://seer.cancer.gov/publications/childhood/sympathetic.pdf (accessed 8 July 2013).
GPOH 2004
-
- Gesellschaft für Pädiatrische Onkologie und Hämatologie. NB2004 trial protocol for risk adapted treatment of children with neuroblastoma. http://www.kinderkrebsinfo.de/sites/kinderkrebsinfo/content/e1676/e9032/... 2004; Vol. Version: 1.00 (01.09.2004); accessed July 8, 2013.
GRADEpro 2008 [Computer program]
-
- Information Management System (IMS), The Nordic Cochrane Centre, The Cochrane Collaboration. GRADEpro (GRADE profiler). Version 3.2 for Windows. Copenhagen: Information Management System (IMS), The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Hero 2008
-
- Hero B, Simon T, Spitz R, Ernestus K, Gnekow AK, Scheel‐Walter HG, et al. Localized infant neuroblastomas often show spontaneous regression: results of the prospective trials NB95‐S and NB97. Journal of Clinical Oncology 2008;26(9):1504‐10. - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
ICSSC 2003
-
- International Clinical Studies Support Center (ICSSC). WHO Toxicity Grading Scale for Determining The Severity of Adverse Events. http://www.icssc.org/Documents/Resources/AEManual2003AppendicesFebruary_... (accessed 25 September 2013.
ICTRP 2014
-
- ICTRP International Clinical Trials Registry Platform, WHO World Health Organization. ICTRP Search Platform. http://apps.who.int/trialsearch/.
Kreissman 2013
Kremer 2008
-
- Kremer LCM, Dalen EC, Moher D, Caron HN. Cochrane Childhood Cancer Group. About The Cochrane Collaboration 2008, Issue 3. Art. No.: CHILDCA.
Kushner 1994
-
- Kushner BH, LaQuaglia MP, Bonilla MA, Lindsley K, Rosenfield N, Yeh S, et al. Highly effective induction therapy for stage 4 neuroblastoma in children over 1 year of age. Journal of Clinical Oncology 1994;12(12):2607‐13. - PubMed
Kushner 2004
-
- Kushner BH, Kramer K, LaQuaglia MP, Modak S, Yataghene K, Cheung NK. Reduction from seven to five cycles of intensive induction chemotherapy in children with high‐risk neuroblastoma. Journal of Clinical Oncology 2004;22(24):4888‐92. - PubMed
Lefebvre 2011
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook of Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Matthay 1999
-
- Matthay KK, Villablanca JG, Seeger RC, Stram DO, Harris RE, Ramsay NK, et al. Treatment of high‐risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13‐cis‐retinoic acid. Children's Cancer Group. New England Journal of Medicine 1999;341(16):1165‐73. - PubMed
Matthay 2009
-
- Matthay KK, Reynolds CP, Seeger RC, Shimada H, Adkins ES, Haas‐Kogan D, et al. Long‐term results for children with high‐risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13‐cis‐retinoic acid: a children's oncology group study. Journal of Clinical Oncology 2009;27(7):1007‐13. - PMC - PubMed
Moher 2009
NCI CTCAE 2010
-
- National Cancer Institute (NCI), Bethesda, MD, USA. Common Terminology Criteria for Adverse Events (CTCAE) and Common Toxicity Criteria (CTC). http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm (accessed 25 September 2013).
NCI PDQ 2013
-
- National Cancer Institute (NCI) at the National Institutes of Health (NIH), Physician Data Query (PDQ). Neuroblastoma treatment. http://www.cancer.gov/cancertopics/pdq/treatment/neuroblastoma/HealthPro... (accessed 25 September 2013).
Park 2011
-
- Park JR, Scott JR, Stewart CF, London WB, Naranjo A, Santana VM, et al. Pilot induction regimen incorporating pharmacokinetically guided topotecan for treatment of newly diagnosed high‐risk neuroblastoma: a Children's Oncology Group study. Journal of Clinical Oncology 2011;29(33):4351‐7. - PMC - PubMed
Parmar 1998
-
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. - PubMed
Pearson 2008
-
- Pearson AD, Pinkerton CR, Lewis IJ, Imeson J, Ellershaw C, Machin D, et al. High‐dose rapid and standard induction chemotherapy for patients aged over 1 year with stage 4 neuroblastoma: a randomised trial. Lancet Oncology 2008;9(3):247‐56. - PubMed
Review Manager 2013 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2013.
Tierney 2007
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical

