Gemcitabine Plus Cisplatin for Advanced Biliary Tract Cancer: A Systematic Review
- PMID: 25989801
- PMCID: PMC4509359
- DOI: 10.4143/crt.2014.308
Gemcitabine Plus Cisplatin for Advanced Biliary Tract Cancer: A Systematic Review
Abstract
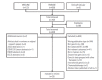
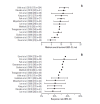
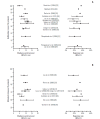
Evidence suggests that combined gemcitabine-cisplatin chemotherapy extends survival in patients with advanced biliary tract cancer (BTC). We conducted a systematic review in order to collate this evidence and assess whether gemcitabine-cisplatin efficacy is influenced by primary tumor site, disease stage, or geographic region, and whether associated toxicities are related to regimen. MEDLINE (1946-search date), EMBASE (1966-search date), ClinicalTrials. gov (2008-search date), and abstracts from major oncology conferences (2009- search date) were searched (5 Dec 2013) using terms for BTC, gemcitabine, and cisplatin. All study types reporting efficacy (survival, response rates) or safety (toxicities) outcomes of gemcitabine-cisplatin in BTC were eligible for inclusion; efficacy data were extracted from prospective studies only. Evidence retrieved from one meta-analysis (abstract), four randomized controlled trials, 12 nonrandomized prospective studies, and three retrospective studies supported the efficacy and safety of gemcitabine-cisplatin for BTC. Median overall survival ranged from 4.6 to 11.7 months, and response rate ranged from 17.1% to 36.6%. Toxicities were generally acceptable and manageable. Heterogeneity in study designs and data collected prevented formal meta-analysis, however exploratory assessments suggested that efficacy did not vary with primary tumor site (gallbladder vs. others), disease stage (metastatic vs. locally advanced), or geographic origin (Asia vs. other). Incidence of grade 3/4 toxicities was not related to gemcitabine dose or cisplatin frequency. Despite individual variation in study designs, the evidence presented suggests that gemcitabine-cisplatin is effective in patients from a diverse range of countries and with heterogeneous disease characteristics. No substantial differences in toxicity were observed among the different dosing schedules of gemcitabine and cisplatin.
Keywords: Biliary tract neoplasms; Cholangiocarcinoma; Cisplatin; Gallbladder neoplasms; Gemcitabine.
Conflict of interest statement
Eli Lilly and Company, manufacturer/licensee of gemicitabine (Gemzar), was involved in the study design, data collection, data analysis, and preparation of the manuscript. Do-Youn Oh has received research funding from Eli Lilly. Jen-Shi Chen has received consultancy fees and honoraria from Eli Lilly, Roche, and Novartis. Li-Tzong Chen has received honoraria from Eli Lilly, Novartis, TTY Biopharm, and PharmaEngine, and support for investigator-initiated trials from Merck Serono, Novartis, Sanofi-Aventis, and TTY. Jong Seok Kim is an employee of and owns stock in Eli Lilly Korea Ltd., Republic of Korea. Mauro Orlando is an employee of and owns stock in Eli Lilly Interamerica, Argentina. Joon Oh Park, Chiun Hsu, and Ho Yeong Lim have no conflicts of interest to declare.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

