Aflibercept versus ranibizumab for treating persistent diabetic macular oedema
- PMID: 25989873
- PMCID: PMC4488481
- DOI: 10.1007/s10792-015-0081-7
Aflibercept versus ranibizumab for treating persistent diabetic macular oedema
Abstract
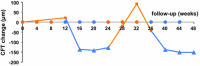
The purpose of this study was to compare the efficacy of intravitreal aflibercept versus ranibizumab for treating therapy-resistant diabetic macular oedema (DME). A 69-year-old man presented with persistent bilateral DME despite previous ranibizumab treatment. Bilateral study treatment comprised one cycle of three monthly ranibizumab injections (0.5 mg), followed by one cycle of three aflibercept injections (2.0 mg), a second ranibizumab cycle and a second aflibercept cycle. Baseline visual acuity (ETDRS score) was 60 letters for the right eye and 65 letters for the left eye. Baseline central foveal thickness (CFT) was 305 μm for the right eye and 453 μm for the left eye. Substantially improved outcomes were observed during the first aflibercept cycle. CFT was reduced by 150 μm (mean) in both the eyes and decreased below the lowest level achieved during the previous 2.5-year ranibizumab treatment. Visual acuity was improved by 17.5 letters (mean) in both the eyes. Reintroduction of ranibizumab immediately worsened the status of both eyes back to the baseline level. During the final aflibercept cycle, visual acuity and CFT improved to the same levels achieved during the first aflibercept cycle. In this case study, we prospectively switched the treatment three times and observed a dramatic and consistent treatment advantage for aflibercept.
Figures
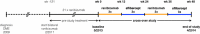


References
-
- Muether PS, Droege KM, Fauser S. Vascular endothelial growth factor suppression times in patients with diabetic macular oedema treated with ranibizumab. Br J Ophthalmol. 2014;98:179–181. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

