Profiling of relaxin and its receptor proteins in boar reproductive tissues and spermatozoa
- PMID: 25990010
- PMCID: PMC4445784
- DOI: 10.1186/s12958-015-0043-y
Profiling of relaxin and its receptor proteins in boar reproductive tissues and spermatozoa
Abstract
Background: Relaxin levels in seminal plasma have been associated with positive effects on sperm motility and quality, and thus having potential roles in male fertility. However, the origin of seminal relaxin, within the male reproductive tract, and the moment of its release in the vicinity of spermatozoa remain unclear. Here, we assessed the longitudinal distribution of relaxin and its receptors RXFP1 and RXFP2 in the reproductive tract, sex accessory glands, and spermatozoa of adult boars.
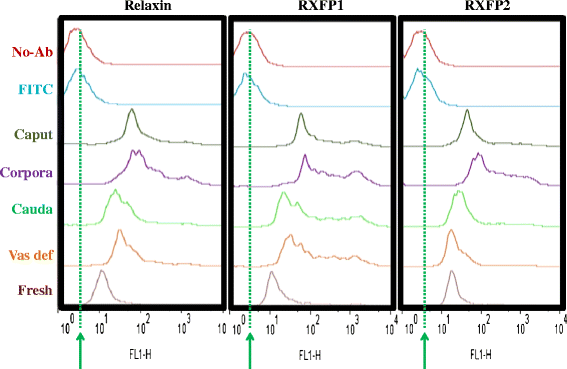
Methods: Spermatozoa were harvested from three fertile boars and reproductive tract (testes and epididymis) and sex accessory gland (prostate and seminal vesicles) tissues were collected post-mortem from each boar. Epididymis ducts were sectioned into caput, corpus, and cauda regions, and spermatozoa were mechanically collected. All samples were subjected to immunofluorescence and/or western immunoblotting for relaxin, RXFP1, and RXFP2 detection. Immunolabeled-spermatozoa were submitted to flow cytometry analyses and data were statistically analyzed with ANOVA.
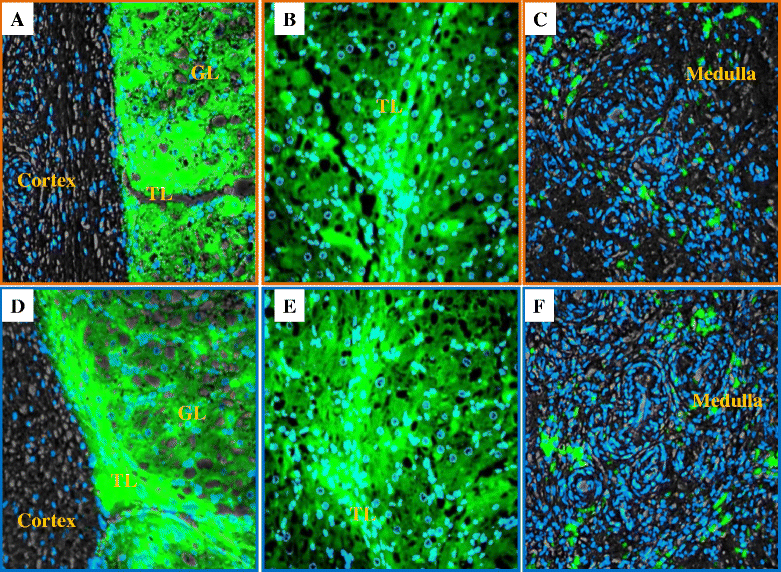
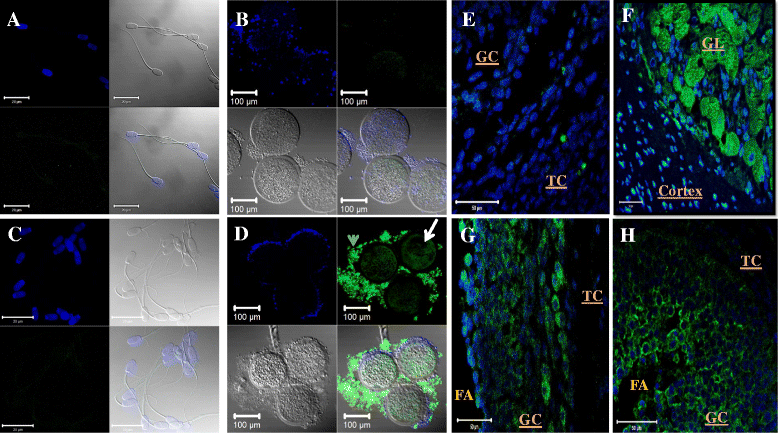
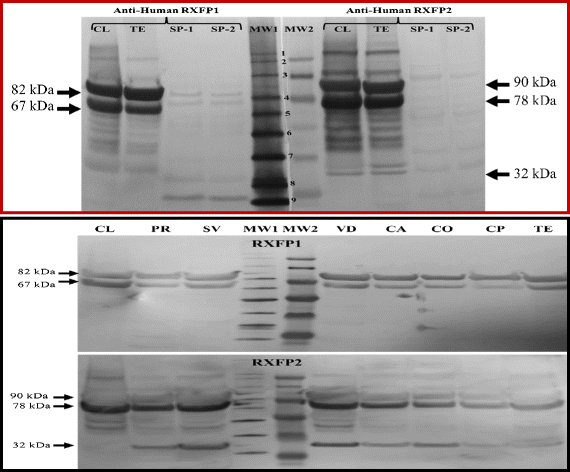
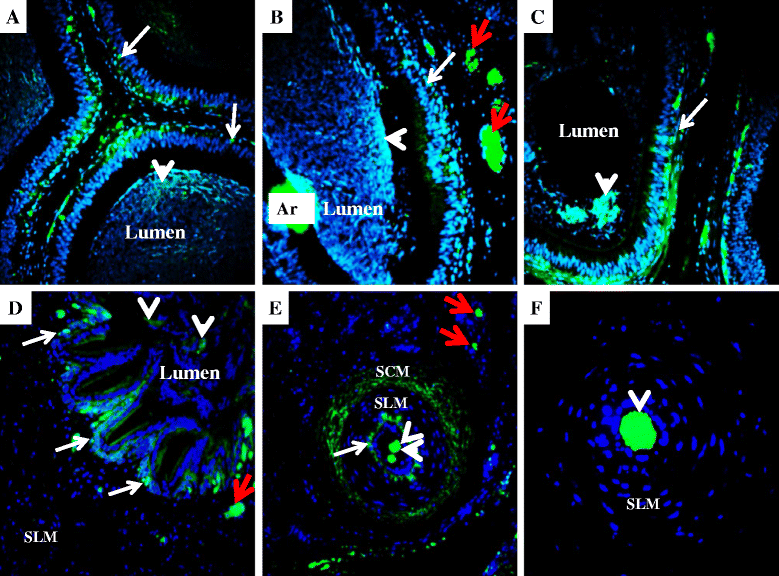
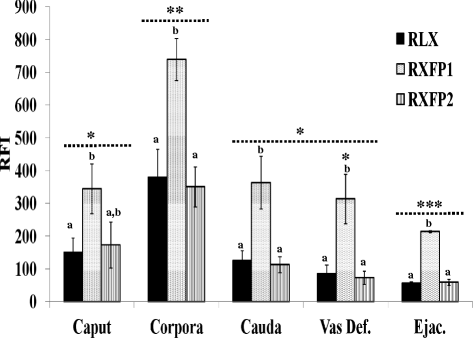
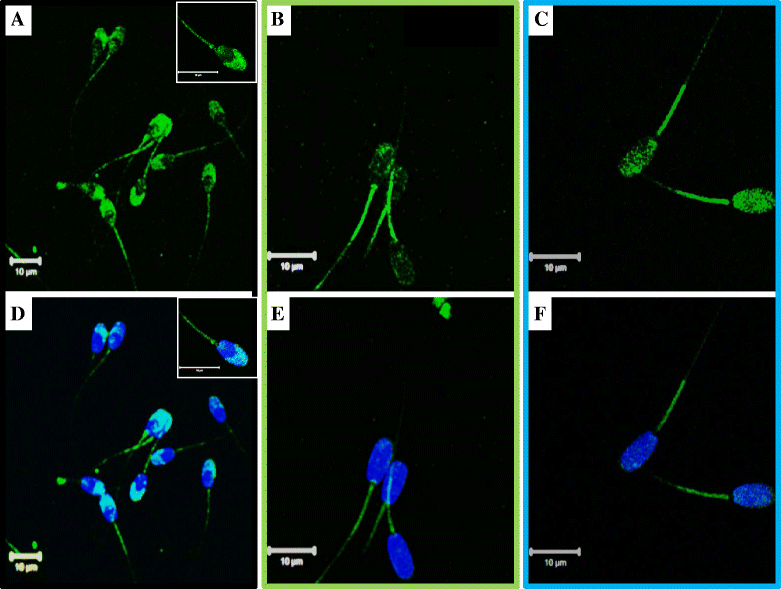
Results: Both receptors were detected in all tissues, with a predominance of mature and immature isoforms of RXFP1 and RXFP2, respectively. Relaxin signals were found in the testes, with Leydig cells displaying the highest intensity compared to other testicular cells. The testicular immunofluorescence intensity of relaxin was greater than that of other tissues. Epithelial basal cells exhibited the highest relaxin immunofluorescence intensity within the epididymis and the vas deferens. The luminal immunoreactivity to relaxin was detected in the seminiferous tubule, epididymis, and vas deferens ducts. Epididymal and ejaculated spermatozoa were immunopositive to relaxin, RXFP1, and RXFP2, and epididymal corpus-derived spermatozoa had the highest immunoreactivities across epididymal sections. Both vas deferens-collected and ejaculated spermatozoa displayed comparable, but lowest immunofluorescence signals among groups. The entire sperm length was immunopositive to both relaxin and receptors, with relaxin signal being robust in the acrosome area and RXFP2, homogeneously distributed than RXFP1 on the head of ejaculated spermatozoa.
Conclusions: Immunolocalization indicates that relaxin-receptor complexes may have important roles in boar reproduction and that spermatozoa are already exposed to relaxin upon their production. The findings suggest autocrine and/or paracrine actions of relaxin on spermatozoa, either before or after ejaculation, which have possible roles on the fertilizing potential of spermatozoa.
Figures



Similar articles
-
Relaxin family peptide receptors Rxfp1 and Rxfp2: mapping of the mRNA and protein distribution in the reproductive tract of the male rat.Reprod Biol Endocrinol. 2007 Jul 10;5:29. doi: 10.1186/1477-7827-5-29. Reprod Biol Endocrinol. 2007. PMID: 17623071 Free PMC article.
-
Beneficial effects of relaxin on motility characteristics of stored boar spermatozoa.Reprod Biol Endocrinol. 2015 Mar 31;13:24. doi: 10.1186/s12958-015-0021-4. Reprod Biol Endocrinol. 2015. PMID: 25880070 Free PMC article.
-
G protein-coupled estrogen receptor (GPER) in adult boar testes, epididymis and spermatozoa during epididymal maturation.Int J Biol Macromol. 2018 Sep;116:113-119. doi: 10.1016/j.ijbiomac.2018.05.015. Epub 2018 May 4. Int J Biol Macromol. 2018. PMID: 29730010
-
Relaxin family peptide receptors--former orphans reunite with their parent ligands to activate multiple signalling pathways.Br J Pharmacol. 2007 Mar;150(6):677-91. doi: 10.1038/sj.bjp.0707140. Epub 2007 Feb 12. Br J Pharmacol. 2007. PMID: 17293890 Free PMC article. Review.
-
Development and use of surgical procedures to bypass selected regions of the mammalian epididymis: effects on sperm maturation.J Reprod Fertil Suppl. 1998;53:183-95. J Reprod Fertil Suppl. 1998. PMID: 10645277 Review.
Cited by
-
Identification of genomic variants causing sperm abnormalities and reduced male fertility.Anim Reprod Sci. 2018 Jul;194:57-62. doi: 10.1016/j.anireprosci.2018.02.007. Epub 2018 Feb 10. Anim Reprod Sci. 2018. PMID: 29454799 Free PMC article. Review.
-
Male Seminal Relaxin Contributes to Induction of the Post-mating Cytokine Response in the Female Mouse Uterus.Front Physiol. 2017 Jun 19;8:422. doi: 10.3389/fphys.2017.00422. eCollection 2017. Front Physiol. 2017. PMID: 28674503 Free PMC article.
-
Expression, Localization of SUMO-1, and Analyses of Potential SUMOylated Proteins in Bubalus bubalis Spermatozoa.Front Physiol. 2017 Jun 13;8:354. doi: 10.3389/fphys.2017.00354. eCollection 2017. Front Physiol. 2017. PMID: 28659810 Free PMC article.
-
In-depth proteomic analysis of boar spermatozoa through shotgun and gel-based methods.BMC Genomics. 2018 Jan 18;19(1):62. doi: 10.1186/s12864-018-4442-2. BMC Genomics. 2018. PMID: 29347914 Free PMC article.
-
Integrating Near-Infrared Spectroscopy and Proteomics for Semen Quality Biosensing.Biosensors (Basel). 2025 Jul 15;15(7):456. doi: 10.3390/bios15070456. Biosensors (Basel). 2025. PMID: 40710106 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

