Cardiovascular safety of linagliptin in type 2 diabetes: a comprehensive patient-level pooled analysis of prospectively adjudicated cardiovascular events
- PMID: 25990013
- PMCID: PMC4465456
- DOI: 10.1186/s12933-015-0215-2
Cardiovascular safety of linagliptin in type 2 diabetes: a comprehensive patient-level pooled analysis of prospectively adjudicated cardiovascular events
Abstract
Background: The cardiovascular (CV) safety of linagliptin was evaluated in subjects with type 2 diabetes (T2DM).
Methods: Pre-specified patient-level pooled analysis of all available double-blind, randomized, controlled trials, ≥ 12 weeks' duration (19 trials, 9459 subjects) of linagliptin versus placebo/active treatment. Primary end point: composite of prospectively adjudicated CV death, non-fatal myocardial infarction, non-fatal stroke, and hospitalization for unstable angina (4P-MACE). Hospitalization for congestive heart failure (CHF) was also evaluated; adjudication of CHF was introduced during the phase 3 program (8 trials; 3314 subjects). 4P-MACE was assessed in placebo-controlled trials (subgroup of 18 trials; 7746 subjects). Investigator-reported events suggestive of CHF from 24 placebo-controlled trials (including trials <12 weeks' duration, 8778 subjects) were also analyzed.
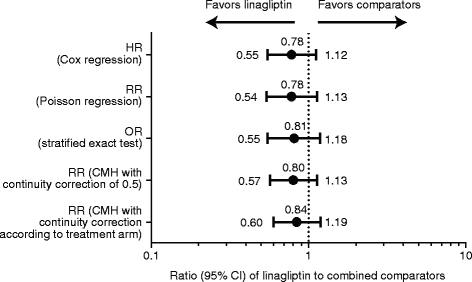
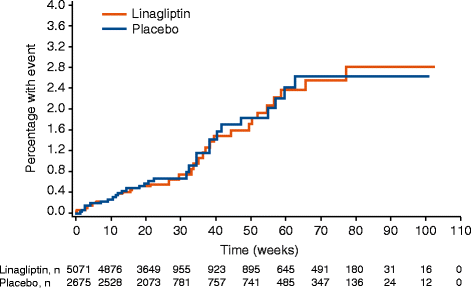
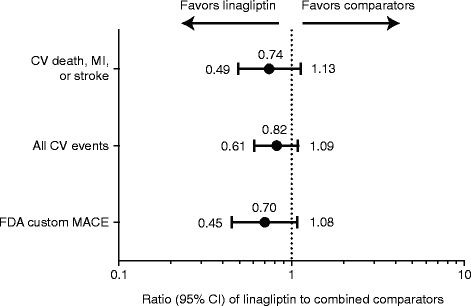
Results: 5847 patients received linagliptin (5 mg: 5687, 10 mg: 160) and 3612 comparator (glimepiride: 775, voglibose: 162, placebo: 2675); cumulative exposure, 4421.3 and 3254.7 patient-years, respectively. 4P-MACE incidence rates: 13.4 per 1000 patient-years, linagliptin (60 events), 18.9, total comparators (62 events); overall hazard ratio (HR), 0.78 (95% confidence interval [CI], 0.55-1.12). HR for adjudicated hospitalization for CHF (n = 21): 1.04 (0.43-2.47). For placebo-controlled trials, 4P-MACE incidence rates: 14.9 per 1000 patient-years, linagliptin (43 events), 16.4, total comparators (29 events); overall HR, 1.09 (95% CI, 0.68-1.75). Occurrence of investigator-reported events suggestive of CHF was low for linagliptin- (26 events, 0.5%; serious: 16 events, 0.3%) and placebo-treated (8 events, 0.2%; serious: 6 events, 0.2%) patients.
Conclusions: Linagliptin is not associated with increased CV risk versus pooled active comparators or placebo in patients with T2DM.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

