Cyclin-dependent kinase 11(p110) (CDK11(p110)) is crucial for human breast cancer cell proliferation and growth
- PMID: 25990212
- PMCID: PMC4438429
- DOI: 10.1038/srep10433
Cyclin-dependent kinase 11(p110) (CDK11(p110)) is crucial for human breast cancer cell proliferation and growth
Abstract
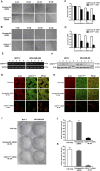
Cyclin-dependent kinases (CDKs) play important roles in the development of many types of cancers by binding with their paired cyclins. However, the function of CDK11 larger protein isomer, CDK11(p110), in the tumorigenesis of human breast cancer remains unclear. In the present study, we explored the effects and molecular mechanisms of CDK11(p110) in the proliferation and growth of breast cancer cells by determining the expression of CDK11(p110) in breast tumor tissues and examining the phenotypic changes of breast cancer cells after CDK11(p110) knockdown. We found that CDK11(p110) was highly expressed in breast tumor tissues and cell lines. Tissue microarray analysis showed that elevated CDK11(p110) expression in breast cancer tissues significantly correlated with poor differentiation, and was also associated with advanced TNM stage and poor clinical prognosis for breast cancer patients. In vitro knockdown of CDK11(p110) by siRNA significantly inhibited cell growth and migration, and dramatically induced apoptosis in breast cancer cells. Flow cytometry demonstrated that cells were markedly arrested in G1 phase of the cell cycle after CDK11(p110) downregulation. These findings suggest that CDK11(p110) is critical for the proliferation and growth of breast cancer cells, which highlights CDK11(p110) may be a promising therapeutic target for the treatment of breast cancer.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





References
-
- Torre L. A. et al. Global cancer statistics, 2012. CA. Cancer J. Clin. 65, 87–108 (2015). - PubMed
-
- DeSantis C., Ma J., Bryan L. & Jemal A. Breast cancer statistics, 2013. CA. Cancer J. Clin. 64, 52–62 (2014). - PubMed
-
- Mohamed A., Krajewski K., Cakar B. & Ma C. X. Targeted therapy for breast cancer. Am. J. Pathol. 183, 1096–1112 (2013). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

