OsTCTP, encoding a translationally controlled tumor protein, plays an important role in mercury tolerance in rice
- PMID: 25990386
- PMCID: PMC4438481
- DOI: 10.1186/s12870-015-0500-y
OsTCTP, encoding a translationally controlled tumor protein, plays an important role in mercury tolerance in rice
Abstract
Background: Mercury (Hg) is not only a threat to public health but also a growth risk factor to plants, as it is readily accumulated by higher plants. Accumulation of Hg in plants disrupts many cellular-level functions and inhibits growth and development; however, the detoxification and tolerance mechanisms of plants to Hg stress are still not fully understood. Exposure to toxic Hg also occurs in some crops cultivated under anoxic conditions, such as rice (Oryza sativa L.), a model organism and one of the most important cultivated plants worldwide. In this study, we functionally characterized a rice translationally controlled tumor protein gene (Os11g43900, OsTCTP) involved in Hg stress tolerance.
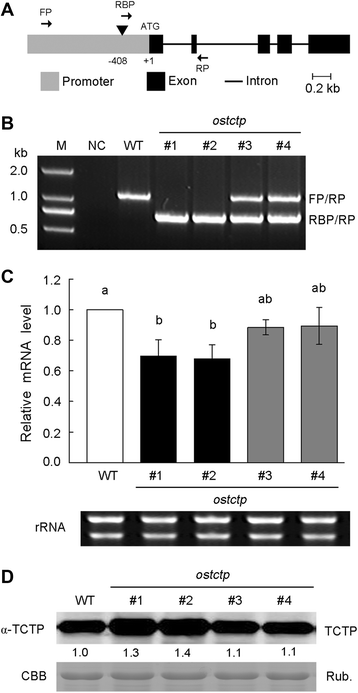
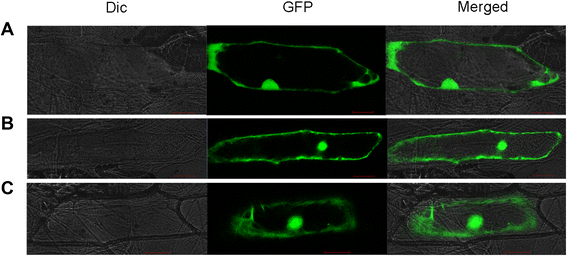
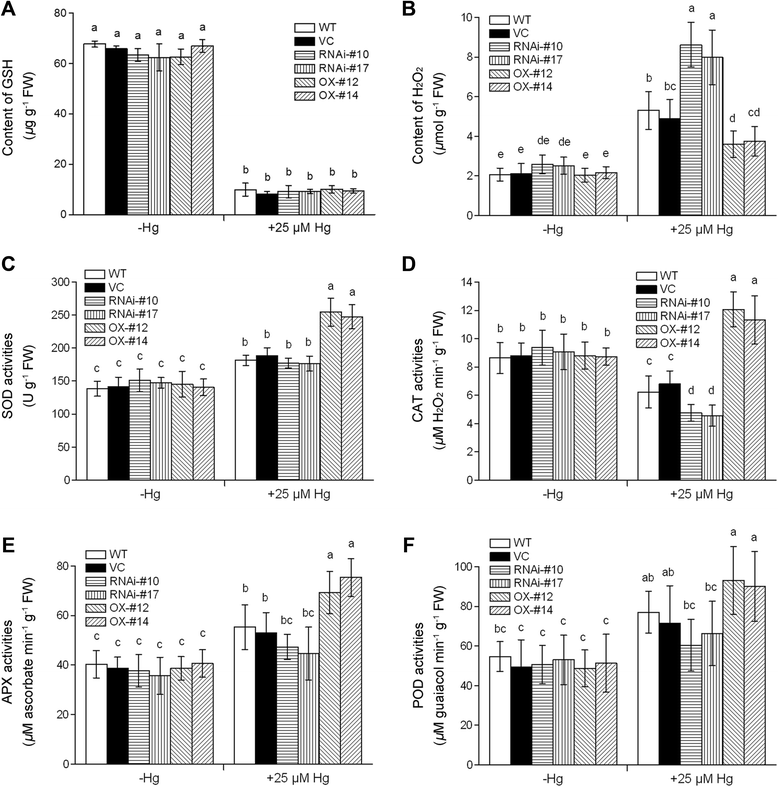
Results: OsTCTP was ubiquitously expressed in all examined plant tissues, especially in actively dividing and differentiating tissues, such as roots and nodes. OsTCTP was found to localize both the cytosol and the nucleus. OsTCTP was induced by mercuric chloride, cupric sulfate, abscisic acid, and hydrogen peroxide at the protein level in a time-dependent manner. Overexpression of OsTCTP potentiated the activities of several antioxidant enzymes, reduced the Hg-induced H2O2 levels, and promoted Hg tolerance in rice, whereas knockdown of OsTCTP produced opposite effects. And overexpression of OsTCTP did not prevent Hg absorption and accumulation in rice. We also demonstrated that Asn 48 and Asn 97 of OsTCTP amino acids were not the potential N-glycosylation sites.
Conclusions: Our results suggest that OsTCTP is capable of decreasing the Hg-induced reactive oxygen species (ROS), therefore, reducing the damage of ROS and enhancing the tolerance of rice plants to Hg stress. Thus, OsTCTP is a valuable gene for genetic engineering to improve rice performance under Hg contaminated paddy soils.
Figures







References
-
- Carrasco-Gil S, Siebner H, Leduc DL, Webb SM, Millán R, Andrews JC, et al. Mercury localization and speciation in plants grown hydroponically or in a natural environment. Environ Sci Technol. 2013;47(7):3082–90. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

