Efficacy and safety of nonsteroidal anti-inflammatory drugs in Asian patients with knee osteoarthritis: summary of a randomized, placebo-controlled study
- PMID: 25990559
- PMCID: PMC5032971
- DOI: 10.1111/1756-185X.12667
Efficacy and safety of nonsteroidal anti-inflammatory drugs in Asian patients with knee osteoarthritis: summary of a randomized, placebo-controlled study
Abstract
Aim: To compare the efficacy, tolerability and safety of celecoxib, naproxen and placebo in Asian patients with osteoarthritis (OA) of the knee.
Method: Patients of Asian descent with knee OA, aged ≥ 45 years, in a flare state with a functional capacity classification of I-III, received celecoxib 200 mg once daily, naproxen 500 mg twice daily or placebo, for 6 weeks. The change in Patient's Assessment of Arthritis Pain (week 6 vs. baseline) was the primary endpoint. Secondary endpoints, including Patient's and Physician's Global Assessments of Arthritis, Western Ontario and McMaster Universities OA Index (WOMAC), use of complementary and alternative medicines, incidence of treatment-emergent adverse events (TEAEs) and measurements of upper gastrointestinal tolerability, were also assessed.
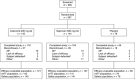
Results: Three hundred and sixty-seven patients were randomized: 145 to celecoxib, 144 to naproxen and 78 to placebo. Celecoxib was as effective as naproxen in reducing OA pain (least squares mean change from baseline in visual analogue scale score [standard error] -37.1 [2.0] for celecoxib and -37.5 [2.0] for naproxen). Patient's and Physician's Global Assessment of Arthritis, WOMAC scores, Pain Satisfaction Scale and Patient Health Questionnaire-9 showed statistically significant improvement in active treatment groups versus placebo, with the exception of naproxen WOMAC scores. Treatment-related TEAEs occurred in 19 (13%), 34 (24%) and six (8%) patients in the celecoxib, naproxen and placebo groups, respectively.
Conclusion: Celecoxib and naproxen were comparable in their effects to reduce the signs and symptoms of knee OA in Asian patients. Celecoxib was shown to be safe and well tolerated in this patient population.
Keywords: cyclooxygenase-2; ethnicity; nonsteroidal anti-inflammatory drugs; race.
© 2015 The Authors. International Journal of Rheumatic Diseases published by Asia Pacific League of Associations for Rheumatology and Wiley Publishing Asia Pty Ltd.
Figures
References
-
- Thomas PA (2007) Racial and ethnic differences in osteoporosis. J Am Acad Orthop Surg 15 (Suppl. 1), S26–30. - PubMed
-
- Fransen M, Bridgett L, March L, Hoy D, Penserga E, Brooks P (2011) The epidemiology of osteoarthritis in Asia. Int J Rheum Dis 14 (2), 113–21. - PubMed
-
- Pew Research Center . US population projections: 2005–2050. Pew Research Hispanic Trends Project website. Available from URL http://www.pewhispanic.org/2008/02/11/us-population-projections-2005-2050/. Accessed 18 August 2014.
-
- Gibson T, Hameed K, Kadir M, Sultana S, Fatima Z, Syed A (1996) Knee pain amongst the poor and affluent in Pakistan. Br J Rheumatol 35, 146–9. - PubMed
-
- Haq SA, Darmawan J, Islam MN et al (2005) Prevalence of rheumatic diseases and associated outcomes in rural and urban communities in Bangladesh: a COPCORD study. J Rheumatol 32, 348–53. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

