The catalytic activity of the kinase ZAP-70 mediates basal signaling and negative feedback of the T cell receptor pathway
- PMID: 25990959
- PMCID: PMC4445242
- DOI: 10.1126/scisignal.2005596
The catalytic activity of the kinase ZAP-70 mediates basal signaling and negative feedback of the T cell receptor pathway
Abstract
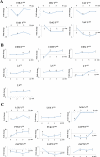
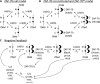
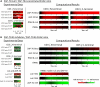
T cell activation by antigens binding to the T cell receptor (TCR) must be properly regulated to ensure normal T cell development and effective immune responses to pathogens and transformed cells while avoiding autoimmunity. The Src family kinase Lck and the Syk family kinase ZAP-70 (ζ chain-associated protein kinase of 70 kD) are sequentially activated in response to TCR engagement and serve as critical components of the TCR signaling machinery that leads to T cell activation. We performed a mass spectrometry-based phosphoproteomic study comparing the quantitative differences in the temporal dynamics of phosphorylation in stimulated and unstimulated T cells with or without inhibition of ZAP-70 catalytic activity. The data indicated that the kinase activity of ZAP-70 stimulates negative feedback pathways that target Lck and thereby modulate the phosphorylation patterns of the immunoreceptor tyrosine-based activation motifs (ITAMs) of the CD3 and ζ chain components of the TCR and of signaling molecules downstream of Lck, including ZAP-70. We developed a computational model that provides a mechanistic explanation for the experimental findings on ITAM phosphorylation in wild-type cells, ZAP-70-deficient cells, and cells with inhibited ZAP-70 catalytic activity. This model incorporated negative feedback regulation of Lck activity by the kinase activity of ZAP-70 and predicted the order in which tyrosines in the ITAMs of TCR ζ chains must be phosphorylated to be consistent with the experimental data.
Copyright © 2015, American Association for the Advancement of Science.
Figures



References
-
- Brownlie RJ, Zamoyska R. T cell receptor signalling networks: branched, diversified and bounded. Nat. Rev. Immunol. 2013;13:257–269. - PubMed
-
- Jordan MS, Singer AL, Koretzky GA. Adaptors as central mediators of signal transduction in immune cells. Nat. Immunol. 2003;4:110–116. - PubMed
-
- Chow LM, Fournel M, Davidson D, Veillette A. Negative regulation of T-cell receptor signalling by tyrosine protein kinase p50csk. Nature. 1993;365:156–160. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

