Measurement of Domain-Specific HER2 (ERBB2) Expression May Classify Benefit From Trastuzumab in Breast Cancer
- PMID: 25991002
- PMCID: PMC4554192
- DOI: 10.1093/jnci/djv136
Measurement of Domain-Specific HER2 (ERBB2) Expression May Classify Benefit From Trastuzumab in Breast Cancer
Abstract
Background: Studies have shown that antibodies targeting the intracellular (ICD) or extracellular domains (ECD) of human epidermal growth factor receptor 2 (HER2) are equivalent when traditional methods are used. We describe a new method to quantify ICD and ECD expression separately and assess the prognostic value of domain-specific HER2 results in patients who received adjuvant trastuzumab therapy.
Methods: We measured HER2 protein expression with quantitative immunofluorescence (QIF) in tissue microarrays (TMA) using two different antibodies targeting the ICD (CB11 and A0485) and ECD (SP3 and D8F12). We assessed the prognostic value of ICD and ECD expression in 180 patients from a clinical trial of adjuvant chemotherapy followed by trastuzumab (HeCOG 10/05). We performed an exploratory univariate domain-specific, disease-free survival (DFS) analysis and compared DFS functions with Kaplan-Meier estimates. All statistical tests were two-sided.
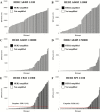
Results: HER2 ICD expression by QIF showed slightly higher sensitivity to predict ERBB2 (HER2) gene amplification than ECD expression, which was more specific and had higher positive predictive value. In the HeCOG 10/05 trial specimens, 15% of cases showed discordant results for ICD and ECD expression. High ECD was statistically associated with longer DFS (log-rank P = .049, HR = 0.31, 95% CI = 0.144 to 0.997), while ICD status was not. Among patients with low ECD, there was no difference in DFS by ICD status. However, when ICD was high, high ECD was statistically associated with longer DFS (log-rank P = .027, HR = 0.23, 95% CI = 0.037 to 0.82) compared with low ECD.
Conclusion: Quantitative measurements of HER2 ICD and ECD expression in breast cancer suggest a subclassification of HER2-positive tumors. Trastuzumab-treated patients with high ECD showed better DFS than patients with low ECD. This suggests differential benefit from trastuzumab therapy based on HER2 ECD expression.
© The Author 2015. Published by Oxford University Press. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures



References
-
- Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31(31):3997–4013. - PubMed
-
- College of American Pathologists. Participant Summary. HER2-A immunohistochemistry Tissues microarray. Surveys 2013 and Anatomic Pathology Education Programs.
-
- Rhodes A, Sarson J, Assam EE, Dean SJ, Cribb EC, Parker A. The reliability of rabbit monoclonal antibodies in the immunohistochemical assessment of estrogen receptors, progesterone receptors, and HER2 in human breast carcinomas. Am J Clin Pathol. 2010;134(4):621–632. - PubMed
-
- Nunes CB, Rocha RM, Buzelin MA, et al. False positivity in HER2 testing of breast cancer: novel paths for approaching an old dilemma. J Clin Pathol. 2013;66(11):946–950. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

