Coronary Wall Structural Changes in Patients With Kawasaki Disease: New Insights From Optical Coherence Tomography (OCT)
- PMID: 25991013
- PMCID: PMC4599424
- DOI: 10.1161/JAHA.115.001939
Coronary Wall Structural Changes in Patients With Kawasaki Disease: New Insights From Optical Coherence Tomography (OCT)
Abstract
Background: Coronary artery aneurysms (CAA) are serious complications of Kawasaki disease (KD). Optical coherence tomography (OCT) is a high-resolution intracoronary imaging modality that characterizes coronary artery wall structure. The purpose of this work was to describe CAA wall sequelae after KD.
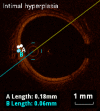
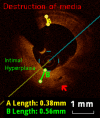
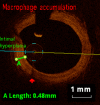
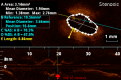
Methods and results: KD patients scheduled for routine coronary angiography underwent OCT imaging between March 2013 and August 2014. Subjects' clinical courses, echocardiography, and coronary angiography examinations were reviewed retrospectively. OCT was performed in 18 patients aged 12.4±5.5 years, 9.0±5.1 years following onset of KD. Of those, 14 patients (77.7%) had a history of CAA (7 with giant CAA and 7 with regressed CAA at time of OCT). Intracoronary nitroglycerin was given to all patients (88.4±45.5 μg/m(2)). Mean radiation dose was 10.9±5.2 mGy/kg. One patient suffered from a transitory uneventful vasospasm at the site of a regressed CAA; otherwise no major procedural complications occurred. The most frequent abnormality observed on OCT was intimal hyperplasia (15 patients, 83.3%) seen at both aneurysmal sites and angiographically normal segments amounting to 390.8±166.0 μm for affected segments compared to 61.7±17 μm for unaffected segments (P<0.001). Disappearance of the media, and presence of fibrosis, calcifications, macrophage accumulation, neovascularization, and white thrombi were seen in 72.2%, 77.8%, 27.8%, 44.4%, and 33.3% of patients.
Conclusions: In this study, OCT proved safe and insightful in the setting of KD, with the potential to add diagnostic value in the assessment of coronary abnormalities in KD. The depicted coronary structural changes correspond to histological findings previously described in KD.
Keywords: coronary disease; imaging; pediatrics.
© 2015 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley Blackwell.
Figures





References
-
- Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, Shulman ST, Bolger AF, Ferrieri P, Baltimore RS, Wilson WR, Baddour LM, Levison ME, Pallasch TJ, Falace DA, Taubert KA. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Pediatrics. 2004;114:1708–1733. - PubMed
-
- JCS Joint Working Group. Ogawa S, Akagi T, Baba K, Fujiwara H, Hamaoka K, Ishii M, Karasawa K, Saji T, Sonobe T, Suzuki A, Ayusawa M, Fukazawa R, Ogino H, Okada T, Echigo S, Nakazawa M, Ochi M, Yamaguchi T. Guidelines for diagnosis and management of cardiovascular sequelae in Kawasaki disease. Circ J. 2010;74:1989–2020. - PubMed
-
- Mitani Y, Ohashi H, Sawada H, Ikeyama Y, Hayakawa H, Takabayashi S, Maruyama K, Shimpo H, Komada Y. In vivo plaque composition and morphology in coronary artery lesions in adolescents and young adults long after Kawasaki disease: a virtual histology-intravascular ultrasound study. Circulation. 2009;119:2829–2836. - PubMed
-
- Mintz GS, Nissen SE, Anderson WD, Bailey SR, Erbel R, Fitzgerald PJ, Pinto FJ, Rosenfield K, Siegel RJ, Tuzcu EM, Yock PG. American College of Cardiology clinical expert consensus document on standards for acquisition, measurement and reporting of intravascular ultrasound studies (IVUS), a report of the American College of Cardiology, Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2001;37:1478–1492. - PubMed
-
- Suzuki A, Yamagishi M, Kimura K, Sugiyama H, Arakaki Y, Kamiya T, Miyatake K. Functional behavior and morphology of the coronary artery wall in patients with Kawasaki disease assessed by intravascular ultrasound. J Am Coll Cardiol. 1996;27:291–296. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

