Randomised clinical trial of snus versus medicinal nicotine among smokers interested in product switching
- PMID: 25991608
- PMCID: PMC4785094
- DOI: 10.1136/tobaccocontrol-2014-052080
Randomised clinical trial of snus versus medicinal nicotine among smokers interested in product switching
Abstract
Background: An essential component of evaluating potential modified risk tobacco products is to determine how consumers use the product and resulting effects on biomarkers of toxicant exposure.
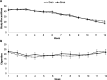
Study design: Cigarette smokers (n=391) recruited in Minnesota and Oregon were randomised to either snus or 4 mg nicotine gum for 12 weeks. Participants were instructed to completely switch from cigarettes to these products. Urine samples were collected to analyse for carcinogenic tobacco-specific nitrosamine metabolites (4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol and N'-nitrosonornicotine and their glucuronides) and nicotine metabolites (total cotinine and nicotine equivalents) levels.
Results: Of the 391 participants randomised, 52.9% were male, the mean±SD age was 43.9±12.5 years, baseline number of cigarettes/day was 18.0±6.5 and Fagerstrom Test for Nicotine Dependence score was 5.1±2.0. The mean±SD number of snus pouches used/week at week 6 prior to tapering was 39.1±24.0 and nicotine gum pieces used was 37.6±26.3. Dual use of cigarettes and these products were observed in 52.9% and 58.2% of those assigned to snus and nicotine gum, respectively, at week 12. The end of treatment biochemically verified (carbon monoxide, CO<6 ppm) 7-day avoidance of cigarettes was 21.9% in the snus group and 24.6% in the nicotine gum group. Toxicant exposure in the nicotine gum group was significantly less when compared to snus.
Conclusions: Snus performed similarly to nicotine gum in cigarette smokers who were interested in completely switching to these products, but was associated with less satisfaction and greater toxicant exposure than nicotine gum.
Trial registration number: NCT: 00710034.
Trial registration: ClinicalTrials.gov NCT00710034.
Keywords: Carcinogens; Harm Reduction; Non-cigarette tobacco products.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Figures
Comment in
-
Rethinking therapeutic and recreational nicotine products: a commentary on Hatsukami et al.Tob Control. 2016 May;25(3):245. doi: 10.1136/tobaccocontrol-2015-052388. Tob Control. 2016. PMID: 27103635 No abstract available.
References
-
- Levy DT, Mumford EA, Cummings KM, et al. The relative risks of a low-nitrosamine smokeless tobacco product compared with smoking cigarettes: estimates of a panel of experts. Cancer Epidemiol Biomarkers Prev 2004;13:2035–42. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical