Mass spectrometry-based microassay of (2)H and (13)C plasma glucose labeling to quantify liver metabolic fluxes in vivo
- PMID: 25991647
- PMCID: PMC4504936
- DOI: 10.1152/ajpendo.00003.2015
Mass spectrometry-based microassay of (2)H and (13)C plasma glucose labeling to quantify liver metabolic fluxes in vivo
Abstract
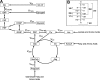
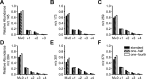
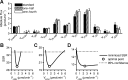
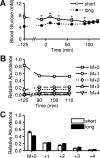
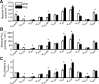
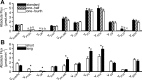
Mouse models designed to examine hepatic metabolism are critical to diabetes and obesity research. Thus, a microscale method to quantitatively assess hepatic glucose and intermediary metabolism in conscious, unrestrained mice was developed. [(13)C3]propionate, [(2)H2]water, and [6,6-(2)H2]glucose isotopes were delivered intravenously in short- (9 h) and long-term-fasted (19 h) C57BL/6J mice. GC-MS and mass isotopomer distribution (MID) analysis were performed on three 40-μl arterial plasma glucose samples obtained during the euglycemic isotopic steady state. Model-based regression of hepatic glucose and citric acid cycle (CAC)-related fluxes was performed using a comprehensive isotopomer model to track carbon and hydrogen atom transitions through the network and thereby simulate the MIDs of measured fragment ions. Glucose-6-phosphate production from glycogen diminished, and endogenous glucose production was exclusively gluconeogenic with prolonged fasting. Gluconeogenic flux from phosphoenolpyruvate (PEP) remained stable, whereas that from glycerol modestly increased from short- to long-term fasting. CAC flux [i.e., citrate synthase (VCS)] was reduced with long-term fasting. Interestingly, anaplerosis and cataplerosis increased with fast duration; accordingly, pyruvate carboxylation and the conversion of oxaloacetate to PEP were severalfold higher than VCS in long-term fasted mice. This method utilizes state-of-the-art in vivo methodology and comprehensive isotopomer modeling to quantify hepatic glucose and intermediary fluxes during physiological stress in mice. The small plasma requirements permit serial sampling without stress and the affirmation of steady-state glucose kinetics. Furthermore, the approach can accommodate a broad range of modeling assumptions, isotope tracers, and measurement inputs without the need to introduce ad hoc mathematical approximations.
Keywords: gluconeogenesis; isotopomer model; liver physiology; metabolic flux analysis; nutrient metabolism.
Copyright © 2015 the American Physiological Society.
Figures

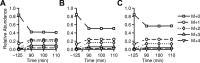

References
-
- Antoniewicz MR, Kelleher JK, Stephanopoulos G. Determination of confidence intervals of metabolic fluxes estimated from stable isotope measurements. Metab Eng 8: 324–337, 2006. - PubMed
-
- Antoniewicz MR, Kelleher JK, Stephanopoulos G. Accurate assessment of amino acid mass isotopomer distributions for metabolic flux analysis. Anal Chem 79: 7554–7559, 2007. - PubMed
-
- Ayala JE, Bracy DP, McGuinness OP, Wasserman DH. Considerations in the design of hyperinsulinemic-euglycemic clamps in the conscious mouse. Diabetes 55: 390–397, 2006. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

