ATP-dependent Conformational Changes Trigger Substrate Capture and Release by an ECF-type Biotin Transporter
- PMID: 25991724
- PMCID: PMC4505438
- DOI: 10.1074/jbc.M115.654343
ATP-dependent Conformational Changes Trigger Substrate Capture and Release by an ECF-type Biotin Transporter
Abstract
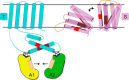
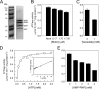
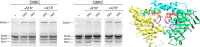
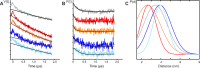
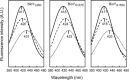
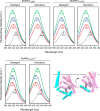
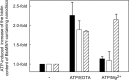
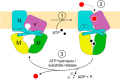
Energy-coupling factor (ECF) transporters for vitamins and metal ions in prokaryotes consist of two ATP-binding cassette-type ATPases, a substrate-specific transmembrane protein (S component) and a transmembrane protein (T component) that physically interacts with the ATPases and the S component. The mechanism of ECF transporters was analyzed upon reconstitution of a bacterial biotin transporter into phospholipid bilayer nanodiscs. ATPase activity was not stimulated by biotin and was only moderately reduced by vanadate. A non-hydrolyzable ATP analog was a competitive inhibitor. As evidenced by cross-linking of monocysteine variants and by site-specific spin labeling of the Q-helix followed by EPR-based interspin distance analyses, closure and reopening of the ATPase dimer (BioM2) was a consequence of ATP binding and hydrolysis, respectively. A previously suggested role of a stretch of small hydrophobic amino acid residues within the first transmembrane segment of the S units for S unit/T unit interactions was structurally and functionally confirmed for the biotin transporter. Cross-linking of this segment in BioY (S) using homobifunctional thiol-reactive reagents to a coupling helix of BioN (T) indicated a reorientation rather than a disruption of the BioY/BioN interface during catalysis. Fluorescence emission of BioY labeled with an environmentally sensitive fluorophore was compatible with an ATP-induced reorientation and consistent with a hypothesized toppling mechanism. As demonstrated by [(3)H]biotin capture assays, ATP binding stimulated substrate capture by the transporter, and subsequent ATP hydrolysis led to substrate release. Our study represents the first experimental insight into the individual steps during the catalytic cycle of an ECF transporter in a lipid environment.
Keywords: ABC transporter; ATP; ECF transporter; biotin; cross-linking; electron paramagnetic resonance (EPR); fluorescence; lipid bilayer nanodiscs; vitamin uptake.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
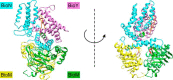


References
-
- Eitinger T., Rodionov D. A., Grote M., Schneider E. (2011) Canonical and ECF-type ATP-binding cassette importers in prokaryotes: diversity in modular organization and cellular functions. FEMS Microbiol. Rev. 35, 3–67 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Research Materials

