Defective Guanine Nucleotide Exchange in the Elongation Factor-like 1 (EFL1) GTPase by Mutations in the Shwachman-Diamond Syndrome Protein
- PMID: 25991726
- PMCID: PMC4505017
- DOI: 10.1074/jbc.M114.626275
Defective Guanine Nucleotide Exchange in the Elongation Factor-like 1 (EFL1) GTPase by Mutations in the Shwachman-Diamond Syndrome Protein
Abstract
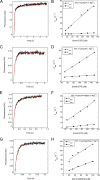
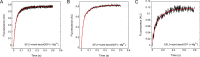
Ribosome biogenesis is orchestrated by the action of several accessory factors that provide time and directionality to the process. One such accessory factor is the GTPase EFL1 involved in the cytoplasmic maturation of the ribosomal 60S subunit. EFL1 and SBDS, the protein mutated in the Shwachman-Diamond syndrome (SBDS), release the anti-association factor eIF6 from the surface of the ribosomal subunit 60S. Here we report a kinetic analysis of fluorescent guanine nucleotides binding to EFL1 alone and in the presence of SBDS using fluorescence stopped-flow spectroscopy. Binding kinetics of EFL1 to both GDP and GTP suggests a two-step mechanism with an initial binding event followed by a conformational change of the complex. Furthermore, the same behavior was observed in the presence of the SBDS protein irrespective of the guanine nucleotide evaluated. The affinity of EFL1 for GTP is 10-fold lower than that calculated for GDP. Association of EFL1 to SBDS did not modify the affinity for GTP but dramatically decreased that for GDP by increasing the dissociation rate of the nucleotide. Thus, SBDS acts as a guanine nucleotide exchange factor (GEF) for EFL1 promoting its activation by the release of GDP. Finally, fluorescence anisotropy measurements showed that the S143L mutation present in the Shwachman-Diamond syndrome altered a surface epitope for EFL1 and largely decreased the affinity for it. These results suggest that loss of interaction between these proteins due to mutations in the disease consequently prevents the nucleotide exchange regulation the SBDS exerts on EFL1.
Keywords: EFL1; GTPase; Shwachman-Diamond syndrome protein; fluorescence anisotropy; fluorescence resonance energy transfer (FRET); guanine nucleotide exchange factor (GEF); guanine nucleotides; ribosome assembly.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures





References
-
- Kressler D., Hurt E., Bassler J. (2010) Driving ribosome assembly. Biochim. Biophys. Acta 1803, 673–683 - PubMed
-
- Thomson E., Ferreira-Cerca S., Hurt E. (2013) Eukaryotic ribosome biogenesis at a glance. J. Cell Sci. 126, 4815–4821 - PubMed
-
- Teng T., Thomas G., Mercer C. A. (2013) Growth control and ribosomopathies. Curr. Opin. Genet. Dev. 23, 63–71 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

