Cumulative, additive benefits of memantine-donepezil combination over component monotherapies in moderate to severe Alzheimer's dementia: a pooled area under the curve analysis
- PMID: 25991927
- PMCID: PMC4436119
- DOI: 10.1186/s13195-015-0109-2
Cumulative, additive benefits of memantine-donepezil combination over component monotherapies in moderate to severe Alzheimer's dementia: a pooled area under the curve analysis
Abstract
Introduction: Treatment in moderate or severe Alzheimer's disease (AD) often involves adding memantine to a cholinesterase-inhibitor (ChEI: donepezil, galantamine, rivastigmine). Evidence from six-month randomized trials and long-term observational studies supports superiority of memantine-ChEI combination to ChEI monotherapy. We utilized area-under-the-curve (AUC) analysis to assess six-month cumulative treatment efficacy of memantine-donepezil combination versus component monotherapies on individual clinical domains and on a composite index.
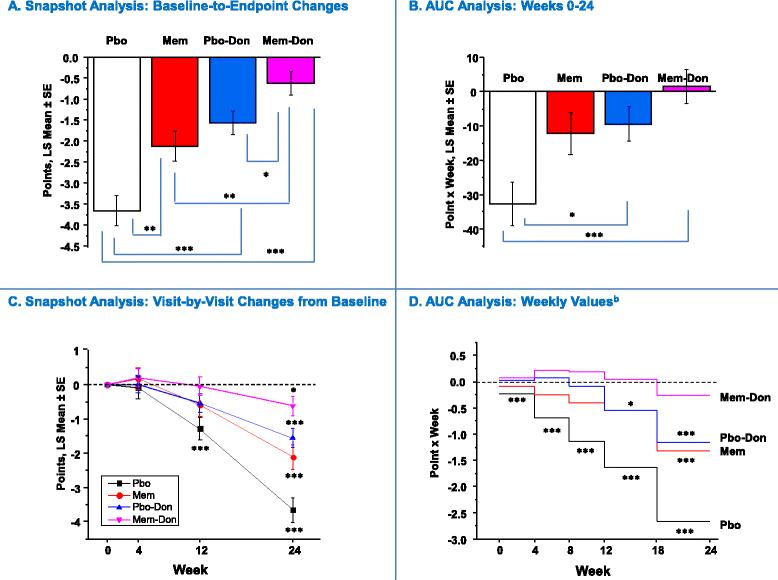
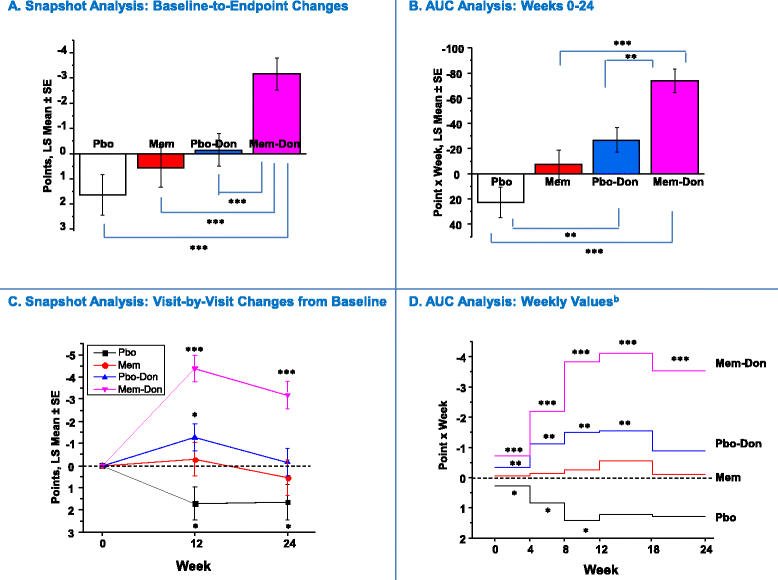
Methods: Data were pooled from 1,408 individuals with moderate to severe AD from four six-month randomized trials of memantine monotherapy (n = 570) or add-on therapy (donepezil-only subset: n = 847). AUC changes from baseline on measures of cognition (SIB), function (ADCS-ADL19), behavior (NPI), global status (CIBIC-Plus), and a composite index (4D-CI: equally weighted composite of four domain measures) were calculated using the trapezoidal rule and evaluated via analysis of covariance (ANCOVA) (2-sided-α = 0.05). AUC results were contrasted with visit-by-visit changes from baseline ("snapshot analysis"), performed using a mixed-effects model with repeated measures (MMRM).
Results: Over the entire six-month period, placebo-only treatment was associated with significant cumulative worsening on all outcomes. Memantine-donepezil combination showed significantly greater AUC improvements (point x week) on the SIB, NPI, and CIBIC-Plus than placebo-donepezil (SIB: 68.4 versus 32.0, P = 0.019; NPI: -74.3 versus -28.2, P = 0.003; CIBIC-Plus: -2.5 versus 1.4, P = 0.006) and memantine-only monotherapies (SIB: 68.4 versus 12.0, P <0.001; NPI: -74.3 versus -7.4, P <0.001; CIBIC-Plus: -2.5 versus 2.7, P <0.001), whereas these comparisons were not significant for the ADCS-ADL19 (memantine-donepezil (1.4) versus placebo-donepezil (-0.9), P = 0.407; versus memantine-only (-12.2), P = 0.310). Composite index analysis demonstrated significant cumulative advantages of memantine-donepezil combination (630.0) over placebo-donepezil (344.7, P <0.001) and memantine-only (152.1, P <0.001) treatments. Combining memantine and donepezil had an additive effect. Compared with AUC analysis, baseline-to-endpoint change-score analysis underestimated effects of combination therapy, monotherapies, or both.
Conclusions: This large pooled area-under-the-curve analysis of randomized-trial data in moderate to severe AD provides ecologically valid support that adding memantine to stable donepezil results in overall clinical benefits that are additive compared with individual monotherapies, continue to accumulate through six-month treatment, and are at least 50% greater than those of monotherapies.
Figures



References
-
- McShane R, Areosa Sastre A, Minakaran N. Memantine for dementia. Cochrane Database Syst Rev. 2006;2:CD003154. - PubMed
-
- Grossberg GT, Manes F, Allegri RF, Gutiérrez-Robledo LM, Gloger S, Xie L, et al. The safety, tolerability, and efficacy of once-daily memantine (28 mg): a multinational, randomized, double-blind, placebo-controlled trial in patients with moderate-to-severe Alzheimer’s disease taking cholinesterase inhibitors. CNS Drugs. 2013;27:469–78. doi: 10.1007/s40263-013-0077-7. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

