A pilot study to assess effects of long-term inhalation of airborne particulate matter on early Alzheimer-like changes in the mouse brain
- PMID: 25992783
- PMCID: PMC4439054
- DOI: 10.1371/journal.pone.0127102
A pilot study to assess effects of long-term inhalation of airborne particulate matter on early Alzheimer-like changes in the mouse brain
Abstract
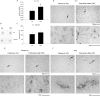
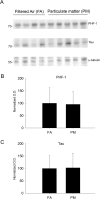
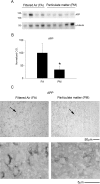
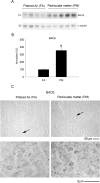
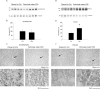
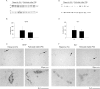
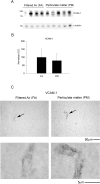
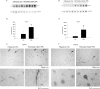
Exposure to air pollutants, including particulate matter, results in activation of the brain inflammatory response and Alzheimer disease (AD)-like pathology in dogs and humans. However, the length of time required for inhalation of ambient particulate matter to influence brain inflammation and AD pathology is less clear. Here, we studied the effect of 3 and 9 months of air particulate matter (<2.5 μm diameter, PM2.5) exposure on brain inflammatory phenotype and pathological hallmarks of AD in C57BL/6 mice. Using western blot, ELISA, and cytokine array analysis we quantified brain APP, beta-site APP cleaving enzyme (BACE), oligomeric protein, total Aβ 1-40 and Aβ 1-42 levels, inducible nitric oxide synthase (iNOS), nitrotyrosine-modified proteins, HNE-Michael adducts, vascular cell adhesion molecule 1 (VCAM-1), glial markers (GFAP, Iba-1), pre- and post- synaptic markers (synaptophysin and PSD-95), cyclooxygenase (COX-1, COX-2) levels, and the cytokine profile in PM2.5 exposed and filtered air control mice. Only 9 month PM2.5 exposure increased BACE protein levels, APP processing, and Aβ 1-40 levels. This correlated with a concomitant increase in COX-1 and COX-2 protein levels and a modest alteration in the cytokine profile. These data support the hypothesis that prolonged exposure to airborne particulate matter has the potential to alter brain inflammatory phenotype and promote development of early AD-like pathology.
Conflict of interest statement
Figures


References
-
- Maslow K, Maslow K, Alzheimer's A 2010 Alzheimer's disease facts and figures. Alzheimer's & dementia: the journal of the Alzheimer's Association. 2010;6: 158–94. - PubMed
-
- Thies W, Bleiler L, Alzheimer's A 2013 Alzheimer's disease facts and figures. Alzheimer's & dementia: the journal of the Alzheimer's Association. 2013;9: 208–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

