Actin depletion initiates events leading to granule secretion at the immunological synapse
- PMID: 25992860
- PMCID: PMC4448150
- DOI: 10.1016/j.immuni.2015.04.013
Actin depletion initiates events leading to granule secretion at the immunological synapse
Abstract
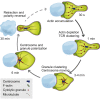
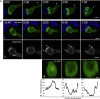
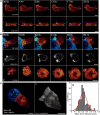
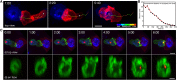
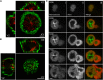
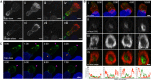
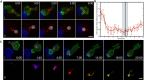
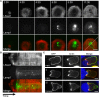
Cytotoxic T lymphocytes (CTLs) use polarized secretion to rapidly destroy virally infected and tumor cells. To understand the temporal relationships between key events leading to secretion, we used high-resolution 4D imaging. CTLs approached targets with actin-rich projections at the leading edge, creating an initially actin-enriched contact with rearward-flowing actin. Within 1 min, cortical actin reduced across the synapse, T cell receptors (TCRs) clustered centrally to form the central supramolecular activation cluster (cSMAC), and centrosome polarization began. Granules clustered around the moving centrosome within 2.5 min and reached the synapse after 6 min. TCR-bearing intracellular vesicles were delivered to the cSMAC as the centrosome docked. We found that the centrosome and granules were delivered to an area of membrane with reduced cortical actin density and phospholipid PIP2. These data resolve the temporal order of events during synapse maturation in 4D and reveal a critical role for actin depletion in regulating secretion.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
Comment in
-
New lamp posts allow for new views of the immunological synapse.Immunity. 2015 May 19;42(5):781-3. doi: 10.1016/j.immuni.2015.05.008. Immunity. 2015. PMID: 25992851
References
-
- Babich A., Burkhardt J.K. Lymphocyte signaling converges on microtubules. Immunity. 2011;34:825–827. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

