Proton Pump Inhibitors Inhibit Pancreatic Secretion: Role of Gastric and Non-Gastric H+/K+-ATPases
- PMID: 25993003
- PMCID: PMC4436373
- DOI: 10.1371/journal.pone.0126432
Proton Pump Inhibitors Inhibit Pancreatic Secretion: Role of Gastric and Non-Gastric H+/K+-ATPases
Abstract
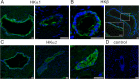
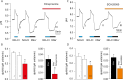
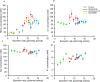
The mechanism by which pancreas secretes high HCO3- has not been fully resolved. This alkaline secretion, formed in pancreatic ducts, can be achieved by transporting HCO3- from serosa to mucosa or by moving H+ in the opposite direction. The aim of the present study was to determine whether H+/K+-ATPases are expressed and functional in human pancreatic ducts and whether proton pump inhibitors (PPIs) have effect on those. Here we show that the gastric HKα1 and HKβ subunits (ATP4A; ATP4B) and non-gastric HKα2 subunits (ATP12A) of H+/K+-ATPases are expressed in human pancreatic cells. Pumps have similar localizations in duct cell monolayers (Capan-1) and human pancreas, and notably the gastric pumps are localized on the luminal membranes. In Capan-1 cells, PPIs inhibited recovery of intracellular pH from acidosis. Furthermore, in rats treated with PPIs, pancreatic secretion was inhibited but concentrations of major ions in secretion follow similar excretory curves in control and PPI treated animals. In addition to HCO3-, pancreas also secretes K+. In conclusion, this study calls for a revision of the basic model for HCO3- secretion. We propose that proton transport is driving secretion, and that in addition it may provide a protective pH buffer zone and K+ recirculation. Furthermore, it seems relevant to re-evaluate whether PPIs should be used in treatment therapies where pancreatic functions are already compromised.
Conflict of interest statement
Figures



References
-
- Novak I, Greger R. Electrophysiological study of transport systems in isolated perfused pancreatic ducts: Properties of the basolateral membrane. Pflügers Arch 1988;411:58–68. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

