In vivo Tracking of Dendritic Cell using MRI Reporter Gene, Ferritin
- PMID: 25993535
- PMCID: PMC4439152
- DOI: 10.1371/journal.pone.0125291
In vivo Tracking of Dendritic Cell using MRI Reporter Gene, Ferritin
Abstract
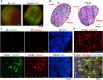
The noninvasive imaging of dendritic cells (DCs) migrated into lymph nodes (LNs) can provide helpful information on designing DCs-based immunotherapeutic strategies. This study is to investigate the influence of transduction of human ferritin heavy chain (FTH) and green fluorescence protein (GFP) genes on inherent properties of DCs, and the feasibility of FTH as a magnetic resonance imaging (MRI) reporter gene to track DCs migration into LNs. FTH-DCs were established by the introduction of FTH and GFP genes into the DC cell line (DC2.4) using lentivirus. The changes in the rate of MRI signal decay (R2*) resulting from FTH transduction were analyzed in cell phantoms as well as popliteal LN of mice after subcutaneous injection of those cells into hind limb foot pad by using a multiple gradient echo sequence on a 9.4 T MR scanner. The transduction of FTH and GFP did not influence the proliferation and migration abilities of DCs. The expression of co-stimulatory molecules (CD40, CD80 and CD86) in FTH-DCs was similar to that of DCs. FTH-DCs exhibited increased iron storage capacity, and displayed a significantly higher transverse relaxation rate (R2*) as compared to DCs in phantom. LNs with FTH-DCs exhibited negative contrast, leading to a high R2* in both in vivo and ex vivo T2*-weighted images compared to DCs. On histological analysis FTH-DCs migrated to the subcapsular sinus and the T cell zone of LN, where they highly expressed CD25 to bind and stimulate T cells. Our study addresses the feasibility of FTH as an MRI reporter gene to track DCs migration into LNs without alteration of their inherent properties. This study suggests that FTH-based MRI could be a useful technique to longitudinally monitor DCs and evaluate the therapeutic efficacy of DC-based vaccines.
Conflict of interest statement
Figures




References
-
- Lucignani G, Rescigno M. The role of molecular imaging in the development of dendritic cell-based cancer vaccines. Eur J Nucl Med Mol Imaging. 2005; 32: 725–730. - PubMed
-
- de Vries IJ, Lesterhuis WJ, Barentsz JO, Verdijk P, van Krieken JH, Boerman OC, et al. Magnetic resonance tracking of dendritic cells in melanoma patients for monitoring of cellular therapy. Nat Biotechnol 2005; 23: 1407–1413. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

