Infarcted Left Ventricles Have Stiffer Material Properties and Lower Stiffness Variation: Three-Dimensional Echo-Based Modeling to Quantify In Vivo Ventricle Material Properties
- PMID: 25994130
- PMCID: PMC4462863
- DOI: 10.1115/1.4030668
Infarcted Left Ventricles Have Stiffer Material Properties and Lower Stiffness Variation: Three-Dimensional Echo-Based Modeling to Quantify In Vivo Ventricle Material Properties
Abstract
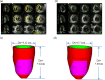
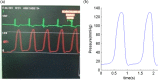
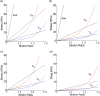
Methods to quantify ventricle material properties noninvasively using in vivo data are of great important in clinical applications. An ultrasound echo-based computational modeling approach was proposed to quantify left ventricle (LV) material properties, curvature, and stress/strain conditions and find differences between normal LV and LV with infarct. Echo image data were acquired from five patients with myocardial infarction (I-Group) and five healthy volunteers as control (H-Group). Finite element models were constructed to obtain ventricle stress and strain conditions. Material stiffening and softening were used to model ventricle active contraction and relaxation. Systolic and diastolic material parameter values were obtained by adjusting the models to match echo volume data. Young's modulus (YM) value was obtained for each material stress-strain curve for easy comparison. LV wall thickness, circumferential and longitudinal curvatures (C- and L-curvature), material parameter values, and stress/strain values were recorded for analysis. Using the mean value of H-Group as the base value, at end-diastole, I-Group mean YM value for the fiber direction stress-strain curve was 54% stiffer than that of H-Group (136.24 kPa versus 88.68 kPa). At end-systole, the mean YM values from the two groups were similar (175.84 kPa versus 200.2 kPa). More interestingly, H-Group end-systole mean YM was 126% higher that its end-diastole value, while I-Group end-systole mean YM was only 29% higher that its end-diastole value. This indicated that H-Group had much greater systole-diastole material stiffness variations. At beginning-of-ejection (BE), LV ejection fraction (LVEF) showed positive correlation with C-curvature, stress, and strain, and negative correlation with LV volume, respectively. At beginning-of-filling (BF), LVEF showed positive correlation with C-curvature and strain, but negative correlation with stress and LV volume, respectively. Using averaged values of two groups at BE, I-Group stress, strain, and wall thickness were 32%, 29%, and 18% lower (thinner), respectively, compared to those of H-Group. L-curvature from I-Group was 61% higher than that from H-Group. Difference in C-curvature between the two groups was not statistically significant. Our results indicated that our modeling approach has the potential to determine in vivo ventricle material properties, which in turn could lead to methods to infer presence of infarct from LV contractibility and material stiffness variations. Quantitative differences in LV volume, curvatures, stress, strain, and wall thickness between the two groups were provided.
Figures




References
-
- Desmond-Hellmann, S. , Sawyers, C. L. , Cox, D. R. , Fraser-Liggett, C. , Galli, S. J. , Goldstein, D. B. , Hunter, D. , Kohane, I. S. , Lo, B. , Misteli, T. , Morrison, S. J. , Nichols, D. G. , Olson, M. V. , Royal, C. D. , and Yamamoto, K. R. , 2011, “Toward Precision Medicine: Building a Knowledge Network for Biomedical Research and a New Taxonomy of Disease,” Committee on a Framework for Development a New Taxonomy of Disease, National Research Council, The National Academies Press.http://www.nap.edu/catalog.php?record_id=13284 - PubMed
-
- McCulloch, A. , Waldman, L. , Rogers, J. , and Guccione, J. , 1992, “Large-Scale Finite Element Analysis of the Beating Heart,” Crit. Rev. Biomed. Eng., 20(5–6), pp. 427–449. - PubMed
-
- Krishnamurthy, A. , Villongco, C. T. , Chuang, J. , Frank, L. R. , Nigam, V. , Belezzuoli, E. , Stark, P. , Krummen, D. E. , Narayan, S. , Omens, J. H. , McCulloch, A. D. , and Kerckhoffs, R. C. , 2013, “Patient-Specific Models of Cardiac Biomechanics,” J. Comput. Phys., 244, pp. 4–21.10.1016/j.jcp.2012.09.015 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

