Large-scale spatiotemporal spike patterning consistent with wave propagation in motor cortex
- PMID: 25994554
- PMCID: PMC4443713
- DOI: 10.1038/ncomms8169
Large-scale spatiotemporal spike patterning consistent with wave propagation in motor cortex
Abstract
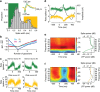
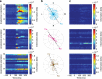
Aggregate signals in cortex are known to be spatiotemporally organized as propagating waves across the cortical surface, but it remains unclear whether the same is true for spiking activity in individual neurons. Furthermore, the functional interactions between cortical neurons are well documented but their spatial arrangement on the cortical surface has been largely ignored. Here we use a functional network analysis to demonstrate that a subset of motor cortical neurons in non-human primates spatially coordinate their spiking activity in a manner that closely matches wave propagation measured in the beta oscillatory band of the local field potential. We also demonstrate that sequential spiking of pairs of neuron contains task-relevant information that peaks when the neurons are spatially oriented along the wave axis. We hypothesize that the spatial anisotropy of spike patterning may reflect the underlying organization of motor cortex and may be a general property shared by other cortical areas.
Figures





Similar articles
-
Primary motor and sensory cortical areas communicate via spatiotemporally coordinated networks at multiple frequencies.Proc Natl Acad Sci U S A. 2016 May 3;113(18):5083-8. doi: 10.1073/pnas.1600788113. Epub 2016 Apr 18. Proc Natl Acad Sci U S A. 2016. PMID: 27091982 Free PMC article.
-
Optogenetically induced spatiotemporal gamma oscillations and neuronal spiking activity in primate motor cortex.J Neurophysiol. 2015 Jun 1;113(10):3574-87. doi: 10.1152/jn.00792.2014. Epub 2015 Mar 11. J Neurophysiol. 2015. PMID: 25761956 Free PMC article.
-
Spatio-Temporal Patterning in Primary Motor Cortex at Movement Onset.Cereb Cortex. 2017 Feb 1;27(2):1491-1500. doi: 10.1093/cercor/bhv327. Cereb Cortex. 2017. PMID: 26759484 Free PMC article.
-
Propagating spatiotemporal activity patterns across macaque motor cortex carry kinematic information.Proc Natl Acad Sci U S A. 2023 Jan 24;120(4):e2212227120. doi: 10.1073/pnas.2212227120. Epub 2023 Jan 18. Proc Natl Acad Sci U S A. 2023. PMID: 36652475 Free PMC article.
-
Phase reorganization leads to transient β-LFP spatial wave patterns in motor cortex during steady-state movement preparation.J Neurophysiol. 2018 Jun 1;119(6):2212-2228. doi: 10.1152/jn.00525.2017. Epub 2018 Feb 14. J Neurophysiol. 2018. PMID: 29442553 Free PMC article.
Cited by
-
Simulation of Code Spectrum and Code Flow of Cultured Neuronal Networks.Comput Intell Neurosci. 2016;2016:7186092. doi: 10.1155/2016/7186092. Epub 2016 Apr 27. Comput Intell Neurosci. 2016. PMID: 27239189 Free PMC article.
-
High-frequency oscillations in human and monkey neocortex during the wake-sleep cycle.Proc Natl Acad Sci U S A. 2016 Aug 16;113(33):9363-8. doi: 10.1073/pnas.1523583113. Epub 2016 Aug 1. Proc Natl Acad Sci U S A. 2016. PMID: 27482084 Free PMC article.
-
Synchronous Spike Patterns in Macaque Motor Cortex during an Instructed-Delay Reach-to-Grasp Task.J Neurosci. 2016 Aug 10;36(32):8329-40. doi: 10.1523/JNEUROSCI.4375-15.2016. J Neurosci. 2016. PMID: 27511007 Free PMC article.
-
Electrocorticographic dissociation of alpha and beta rhythmic activity in the human sensorimotor system.Elife. 2019 Oct 9;8:e48065. doi: 10.7554/eLife.48065. Elife. 2019. PMID: 31596233 Free PMC article.
-
Beta traveling waves in monkey frontal and parietal areas encode recent reward history.Nat Commun. 2023 Sep 5;14(1):5428. doi: 10.1038/s41467-023-41125-9. Nat Commun. 2023. PMID: 37669966 Free PMC article.
References
-
- Arieli A., Shoham D., Hildesheim R. & Grinvald A. Coherent spatiotemporal patterns of ongoing activity revealed by real-time optical imaging coupled with single-unit recording in the cat visual cortex. J Neurophysiol. 73, 2072–2093 (1995). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

