Donor Requirements for Regulatory T Cell Suppression of Murine Graft-versus-Host Disease
- PMID: 25994967
- PMCID: PMC4475671
- DOI: 10.4049/jimmunol.1402861
Donor Requirements for Regulatory T Cell Suppression of Murine Graft-versus-Host Disease
Abstract
Adoptive transfer of freshly isolated natural occurring CD4(+)CD25(+)Foxp3(+) regulatory T cells (Treg) prevents graft-versus-host disease (GVHD) in several animal models and following hematopoietic cell transplantation (HCT) in clinical trials. Donor-derived Treg have been mainly used, as they share the same MHC with CD4(+) and CD8(+) conventional T cells (Tcon) that are primarily responsible for GVHD. Third party-derived Treg are a promising alternative for cellular therapy, as they can be prepared in advance, screened for pathogens and activity, and banked. We explored MHC disparities between Treg and Tcon in HCT to evaluate the impact of different Treg populations in GVHD prevention and survival. Third-party Treg and donor Treg are equally suppressive in ex vivo assays, whereas both donor and third-party but not host Treg protect from GVHD in allogeneic HCT, with donor Treg being the most effective. In an MHC minor mismatched transplantation model (C57BL/6 → BALB/b), donor and third-party Treg were equally effective in controlling GVHD. Furthermore, using an in vivo Treg depletion mouse model, we found that Treg exert their main suppressive activity in the first 2 d after transplantation. Third-party Treg survive for a shorter period of time after adoptive transfer, but despite the shorter survival, they control Tcon proliferation in the early phases of HCT. These studies provide relevant insights on the mechanisms of Treg-mediated protection from GVHD and support for the use of third-party Treg in clinical trials.
Copyright © 2015 by The American Association of Immunologists, Inc.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


 ), Tcon and donor type C57BL/6 Treg (◆), Tcon and third-party FVB/N Treg (◇) and Tcon and host type BALB/C Treg (△) are shown. E: Images of representative animals from each group (minimum 5 mice/group) are shown. For statistical analysis 2-tailed student t test was used, * p < 0.05, *** p < 0.001, ns = not significant. Data are representative of 3 independent experiments.
), Tcon and donor type C57BL/6 Treg (◆), Tcon and third-party FVB/N Treg (◇) and Tcon and host type BALB/C Treg (△) are shown. E: Images of representative animals from each group (minimum 5 mice/group) are shown. For statistical analysis 2-tailed student t test was used, * p < 0.05, *** p < 0.001, ns = not significant. Data are representative of 3 independent experiments.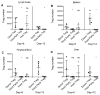


 ), Tcon and donor type C57BL/6 Treg (◆), Tcon and third-party FVB/N Treg (◇) and Tcon and host type BALB/c Treg (△) are shown. Mice that received TCD BM only (dashed line, ■) and mice that were lethally irradiated but not transplanted (dotted line, ●) were used as controls. For statistical analysis of mouse survival Kaplan-Meier test was used, for weight variation and GvHD score 2-way ANOVA test was used, * p < 0.05, ** p < 0.01, *** p < 0.001. Data is representative of one of three experiments (minimum 5 mice/group).
), Tcon and donor type C57BL/6 Treg (◆), Tcon and third-party FVB/N Treg (◇) and Tcon and host type BALB/c Treg (△) are shown. Mice that received TCD BM only (dashed line, ■) and mice that were lethally irradiated but not transplanted (dotted line, ●) were used as controls. For statistical analysis of mouse survival Kaplan-Meier test was used, for weight variation and GvHD score 2-way ANOVA test was used, * p < 0.05, ** p < 0.01, *** p < 0.001. Data is representative of one of three experiments (minimum 5 mice/group).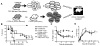
 ), Tcon and donor type C57BL/6 Treg (◆) and Tcon and third-party FVB/N Treg (◇) are shown. Mice that received TCD BM only (dashed line, ■) and mice that were lethally irradiated but not transplanted (dotted line, ●) were used as controls. For statistical analysis of mouse survival Kaplan-Meier test was used, for weight variation and GvHD score 2-way ANOVA test was used, * p < 0.05, ns = not significant. Pooled data from two experiments are shown.
), Tcon and donor type C57BL/6 Treg (◆) and Tcon and third-party FVB/N Treg (◇) are shown. Mice that received TCD BM only (dashed line, ■) and mice that were lethally irradiated but not transplanted (dotted line, ●) were used as controls. For statistical analysis of mouse survival Kaplan-Meier test was used, for weight variation and GvHD score 2-way ANOVA test was used, * p < 0.05, ns = not significant. Pooled data from two experiments are shown.
 ), Tcon + C57BL/6 albino FoxP3DTR/GFP/luc Treg + DT at day −2 and −1 (▽), Tcon + Treg + DT at day 0 and +1 (◇) and Tcon + Treg with no DT (◆) are shown. Mice that received TCD BM only (dashed line, ■) and mice that were lethally irradiated but not transplanted (dotted line, ●) were used as controls. For statistical analysis of mouse survival Kaplan-Meier test was used, for weight variation and GvHD score 2-way ANOVA test was used, * p < 0.05, ns = not significant. Pooled data from two experiments are shown.
), Tcon + C57BL/6 albino FoxP3DTR/GFP/luc Treg + DT at day −2 and −1 (▽), Tcon + Treg + DT at day 0 and +1 (◇) and Tcon + Treg with no DT (◆) are shown. Mice that received TCD BM only (dashed line, ■) and mice that were lethally irradiated but not transplanted (dotted line, ●) were used as controls. For statistical analysis of mouse survival Kaplan-Meier test was used, for weight variation and GvHD score 2-way ANOVA test was used, * p < 0.05, ns = not significant. Pooled data from two experiments are shown.References
-
- Ferrara JL, Deeg HJ. Graft-versus-host disease. The New England journal of medicine. 1991;324:667–674. - PubMed
-
- Jones SC, Murphy GF, Korngold R. Post-hematopoietic cell transplantation control of graft-versus-host disease by donor CD425 T cells to allow an effective graft-versus-leukemia response. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2003;9:243–256. - PubMed
-
- Edinger M, Hoffmann P, Ermann J, Drago K, Fathman CG, Strober S, Negrin RS. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nature medicine. 2003;9:1144–1150. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

