The three 'P's of mitophagy: PARKIN, PINK1, and post-translational modifications
- PMID: 25995186
- PMCID: PMC4441056
- DOI: 10.1101/gad.262758.115
The three 'P's of mitophagy: PARKIN, PINK1, and post-translational modifications
Abstract
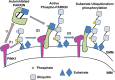
Two Parkinson's disease (PD)-associated proteins, the mitochondrial kinase PINK1 and the E3-ubiquitin (Ub) ligase PARKIN, are central to mitochondrial quality control. In this pathway, PINK1 accumulates on defective mitochondria, eliciting the translocation of PARKIN from the cytosol to mediate the clearance of damaged mitochondria via autophagy (mitophagy). Throughout the different stages of mitophagy, post-translational modifications (PTMs) are critical for the regulation of PINK1 and PARKIN activity and function. Indeed, activation and recruitment of PARKIN onto damaged mitochondria involves PINK1-mediated phosphorylation of both PARKIN and Ub. Through a stepwise cascade, PARKIN is converted from an autoinhibited enzyme into an active phospho-Ub-dependent E3 ligase. Upon activation, PARKIN ubiquitinates itself in concert with many different mitochondrial substrates. The Ub conjugates attached to these substrates can in turn be phosphorylated by PINK1, which triggers further cycles of PARKIN recruitment and activation. This feed-forward amplification loop regulates both PARKIN activity and mitophagy. However, the precise steps and sequence of PTMs in this cascade are only now being uncovered. For instance, the Ub conjugates assembled by PARKIN consist predominantly of noncanonical K6-linked Ub chains. Moreover, these modifications are reversible and can be disassembled by deubiquitinating enzymes (DUBs), including Ub-specific protease 8 (USP8), USP15, and USP30. However, PINK1-mediated phosphorylation of Ub can impede the activity of these DUBs, adding a new layer of complexity to the regulation of PARKIN-mediated mitophagy by PTMs. It is therefore evident that further insight into how PTMs regulate the PINK1-PARKIN pathway will be critical for our understanding of mitochondrial quality control.
Keywords: PARKIN; PINK1; deubiquitination; mitophagy; phosphorylation; ubiquitin.
© 2015 Durcan and Fon; Published by Cold Spring Harbor Laboratory Press.
Figures


References
-
- Bender A, Krishnan KJ, Morris CM, Taylor GA, Reeve AK, Perry RH, Jaros E, Hersheson JS, Betts J, Klopstock T, et al. 2006. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat Genet 38: 515–517. - PubMed
-
- Ben-Saadon R, Zaaroor D, Ziv T, Ciechanover A. 2006. The polycomb protein Ring1B generates self atypical mixed ubiquitin chains required for its in vitro histone H2A ligase activity. Mol Cell 24: 701–711. - PubMed
-
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. 2000. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci 3: 1301–1306. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
