Interferon Beta and Interferon Alpha 2a Differentially Protect Head and Neck Cancer Cells from Vesicular Stomatitis Virus-Induced Oncolysis
- PMID: 25995245
- PMCID: PMC4505650
- DOI: 10.1128/JVI.00757-15
Interferon Beta and Interferon Alpha 2a Differentially Protect Head and Neck Cancer Cells from Vesicular Stomatitis Virus-Induced Oncolysis
Abstract
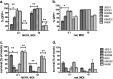
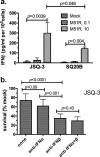
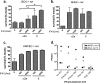
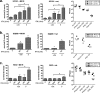
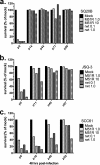
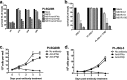
Oncolytic viruses (OV) preferentially kill cancer cells due in part to defects in their antiviral responses upon exposure to type I interferons (IFNs). However, IFN responsiveness of some tumor cells confers resistance to OV treatment. The human type I IFNs include one IFN-β and multiple IFN-α subtypes that share the same receptor but are capable of differentially inducing biological responses. The role of individual IFN subtypes in promoting tumor cell resistance to OV is addressed here. Two human IFNs which have been produced for clinical use, IFN-α2a and IFN-β, were compared for activity in protecting human head and neck squamous cell carcinoma (HNSCC) lines from oncolysis by vesicular stomatitis virus (VSV). Susceptibility of HNSCC lines to killing by VSV varied. VSV infection induced increased production of IFN-β in resistant HNSCC cells. When added exogenously, IFN-β was significantly more effective at protecting HNSCC cells from VSV oncolysis than was IFN-α2a. In contrast, normal keratinocytes and endothelial cells were protected equivalently by both IFN subtypes. Differential responsiveness of tumor cells to IFN-α and -β was further supported by the finding that autocrine IFN-β but not IFN-α promoted survival of HNSCC cells during persistent VSV infection. Therefore, IFN-α and -β differentially affect VSV oncolysis, justifying the evaluation and comparison of IFN subtypes for use in combination with VSV therapy. Pairing VSV with IFN-α2a may enhance selectivity of oncolytic VSV therapy for HNSCC by inhibiting VSV replication in normal cells without a corresponding inhibition in cancer cells.
Importance: There has been a great deal of progress in the development of oncolytic viruses. However, a major problem is that individual cancers vary in their sensitivity to oncolytic viruses. In many cases this is due to differences in their production and response to interferons (IFNs). The experiments described here compared the responses of head and neck squamous cell carcinoma cell lines to two IFN subtypes, IFN-α2a and IFN-β, in protection from oncolytic vesicular stomatitis virus. We found that IFN-α2a was significantly less protective for cancer cells than was IFN-β, whereas normal cells were equivalently protected by both IFNs. These results suggest that from a therapeutic standpoint, selectivity for cancer versus normal cells may be enhanced by pairing VSV with IFN-α2a.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures
Similar articles
-
Employing the Oncolytic Vesicular Stomatitis Virus in Cancer Virotherapy: Resistance and Clinical Considerations.Viruses. 2024 Dec 25;17(1):16. doi: 10.3390/v17010016. Viruses. 2024. PMID: 39861805 Free PMC article. Review.
-
Sensitivity of cervical carcinoma cells to vesicular stomatitis virus-induced oncolysis: potential role of human papilloma virus infection.Int J Cancer. 2012 Aug 1;131(3):E204-15. doi: 10.1002/ijc.27404. Epub 2012 Jan 11. Int J Cancer. 2012. PMID: 22173567
-
Overcoming cancer cell resistance to VSV oncolysis with JAK1/2 inhibitors.Cancer Gene Ther. 2013 Oct;20(10):582-9. doi: 10.1038/cgt.2013.55. Epub 2013 Sep 13. Cancer Gene Ther. 2013. PMID: 24030211 Free PMC article.
-
Resistance of pancreatic cancer cells to oncolytic vesicular stomatitis virus: role of type I interferon signaling.Virology. 2013 Feb 5;436(1):221-34. doi: 10.1016/j.virol.2012.11.014. Epub 2012 Dec 14. Virology. 2013. PMID: 23246628 Free PMC article.
-
VSV-tumor selective replication and protein translation.Oncogene. 2005 Nov 21;24(52):7710-9. doi: 10.1038/sj.onc.1209042. Oncogene. 2005. PMID: 16299531 Review.
Cited by
-
Trastuzumab in combination with PEGylated interferon-α1b exerts synergistic antitumor activity through enhanced inhibition of HER2 downstream signaling and antibody-dependent cellular cytotoxicity.Am J Cancer Res. 2022 Feb 15;12(2):549-561. eCollection 2022. Am J Cancer Res. 2022. PMID: 35261786 Free PMC article.
-
Engineered Oncolytic Poliovirus PVSRIPO Subverts MDA5-Dependent Innate Immune Responses in Cancer Cells.J Virol. 2018 Sep 12;92(19):e00879-18. doi: 10.1128/JVI.00879-18. Print 2018 Oct 1. J Virol. 2018. PMID: 29997212 Free PMC article.
-
VSV based virotherapy in ovarian cancer: the past, the present and …future?J Cancer. 2017 Jul 22;8(12):2369-2383. doi: 10.7150/jca.19473. eCollection 2017. J Cancer. 2017. PMID: 28819441 Free PMC article. Review.
-
Role of β-Interferon Inducer (DEAE-Dextran) in Tumorigenesis by VEGF and NOTCH1 Inhibition along with Apoptosis Induction.Front Pharmacol. 2017 Dec 19;8:930. doi: 10.3389/fphar.2017.00930. eCollection 2017. Front Pharmacol. 2017. PMID: 29311933 Free PMC article.
-
Employing the Oncolytic Vesicular Stomatitis Virus in Cancer Virotherapy: Resistance and Clinical Considerations.Viruses. 2024 Dec 25;17(1):16. doi: 10.3390/v17010016. Viruses. 2024. PMID: 39861805 Free PMC article. Review.
References
-
- Stojdl DF, Lichty BD, ten Oever BR, Paterson JM, Power AT, Knowles S, Marius R, Reynard J, Poliquin L, Atkins H, Brown EG, Durbin RK, Durbin JE, Hiscott J, Bell JC. 2003. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell 4:263–275. doi:10.1016/S1535-6108(03)00241-1. - DOI - PubMed
-
- Colamonici OR, Domanski P, Platanias LC, Diaz MO. 1992. Correlation between interferon (IFN) alpha resistance and deletion of the IFN alpha/beta genes in acute leukemia cell lines suggests selection against the IFN system. Blood 80:744–749. - PubMed
-
- Sun WH, Pabon C, Alsayed Y, Huang PP, Jandeska S, Uddin S, Platanias LC, Rosen ST. 1998. Interferon-alpha resistance in a cutaneous T-cell lymphoma cell line is associated with lack of STAT1 expression. Blood 91:570–576. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

