Tick Saliva Enhances Powassan Virus Transmission to the Host, Influencing Its Dissemination and the Course of Disease
- PMID: 25995246
- PMCID: PMC4505606
- DOI: 10.1128/JVI.01056-15
Tick Saliva Enhances Powassan Virus Transmission to the Host, Influencing Its Dissemination and the Course of Disease
Abstract
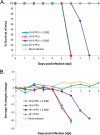
Powassan virus (POWV) is an encephalitic tick-borne flavivirus which can result in serious neuroinvasive disease with up to a 10% case fatality rate. The study objective was to determine whether the salivary gland extract (SGE) from Ixodes scapularis ticks facilitates the transmission and dissemination of POWV in a process known as saliva-activated transmission. Groups of BALB/c mice were footpad inoculated with either a high dose of POWV with and without SGE or a low dose of POWV with and without SGE. Mice from each group were sacrificed daily. Organ viral loads and gene expression profiles were evaluated by quantitative real-time PCR. Both groups of mice infected with high-dose POWV showed severe neurological signs of disease preceding death. The presence of SGE did not affect POWV transmission or disease outcome for mice infected with the high dose of POWV. Neuroinvasion, paralysis, and death occurred for all mice infected with the low dose of POWV plus SGE; however, for mice infected with the low dose of POWV in the absence of SGE, there were no clinical signs of infection and no mice succumbed to disease. Although this group displayed low-level viremias, all mice were completely healthy, and it was the only group in which POWV was cleared from the lymph nodes. We conclude that saliva-activated transmission occurs in mice infected with a low dose of POWV. Our study is the first to demonstrate virus dose-dependent saliva-activated transmission, warranting further investigation of the specific salivary factors responsible for enhancing POWV transmission.
Importance: Powassan virus (POWV) is a tick-borne flavivirus that continues to emerge in the United States, as is evident by the surge in number and expanding geographic range of confirmed cases in the past decade. This neuroinvasive virus is transmitted to humans by infected tick bites. Successful tick feeding is facilitated by a collection of pharmacologically active factors in tick saliva. In a process known as saliva-activated transmission, tick bioactive salivary molecules are thought to modulate the host environment, making it more favorable for the transmission and establishment of a pathogen. This phenomenon has been demonstrated for several tick-borne pathogens; however, a systematic investigation of the role of tick saliva on dissemination and pathogenesis of a tick-borne viral disease has never been attempted before. This study will fill that gap by systematically examining whether the presence of tick saliva contributes to the transmission and dissemination of POWV in mice.
Copyright © 2015, Hermance and Thangamani.
Figures

Similar articles
-
Salivary gland extract from the deer tick, Ixodes scapularis, facilitates neuroinvasion by Powassan virus in BALB/c mice.Sci Rep. 2021 Oct 22;11(1):20873. doi: 10.1038/s41598-021-00021-2. Sci Rep. 2021. PMID: 34686683 Free PMC article.
-
Ixodes scapularis salivary gland microRNAs are differentially expressed during Powassan virus transmission.Sci Rep. 2019 Sep 11;9(1):13110. doi: 10.1038/s41598-019-49572-5. Sci Rep. 2019. PMID: 31511580 Free PMC article.
-
Interspecies co-feeding transmission of Powassan virus between a native tick, Ixodes scapularis, and the invasive East Asian tick, Haemaphysalis longicornis.Parasit Vectors. 2024 Jun 15;17(1):259. doi: 10.1186/s13071-024-06335-0. Parasit Vectors. 2024. PMID: 38879603 Free PMC article.
-
Powassan Virus: An Emerging Arbovirus of Public Health Concern in North America.Vector Borne Zoonotic Dis. 2017 Jul;17(7):453-462. doi: 10.1089/vbz.2017.2110. Epub 2017 May 12. Vector Borne Zoonotic Dis. 2017. PMID: 28498740 Free PMC article. Review.
-
Pathogenicity and virulence of Powassan virus.Virulence. 2025 Dec;16(1):2523887. doi: 10.1080/21505594.2025.2523887. Epub 2025 Jun 26. Virulence. 2025. PMID: 40545598 Free PMC article. Review.
Cited by
-
Discovery of Exosomes From Tick Saliva and Salivary Glands Reveals Therapeutic Roles for CXCL12 and IL-8 in Wound Healing at the Tick-Human Skin Interface.Front Cell Dev Biol. 2020 Jul 16;8:554. doi: 10.3389/fcell.2020.00554. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32766239 Free PMC article.
-
Vertebrate Host Susceptibility to Heartland Virus.Emerg Infect Dis. 2016 Dec;22(12):2070-2077. doi: 10.3201/eid2212.160472. Emerg Infect Dis. 2016. PMID: 27869591 Free PMC article.
-
Arboviruses: How Saliva Impacts the Journey from Vector to Host.Int J Mol Sci. 2021 Aug 25;22(17):9173. doi: 10.3390/ijms22179173. Int J Mol Sci. 2021. PMID: 34502092 Free PMC article. Review.
-
Transcriptional Immunoprofiling at the Tick-Virus-Host Interface during Early Stages of Tick-Borne Encephalitis Virus Transmission.Front Cell Infect Microbiol. 2017 Dec 1;7:494. doi: 10.3389/fcimb.2017.00494. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 29250492 Free PMC article.
-
Modeling Powassan virus infection in Peromyscus leucopus, a natural host.PLoS Negl Trop Dis. 2017 Jan 31;11(1):e0005346. doi: 10.1371/journal.pntd.0005346. eCollection 2017 Jan. PLoS Negl Trop Dis. 2017. PMID: 28141800 Free PMC article.
References
-
- Hinten S, Beckett GA, Gensheimer KF, Pritchard E, Courtney TM, Sears SD, Woytowicz JM, Preston DG, Smith RP Jr, Rand PW, Lacombe EH, Holman MS, Lubelczyk CB, Kelso PT, Beelen AP, Stobierski MG, Sotir MJ, Wong S, Ebel G, Kosoy O, Piesman J, Campbell GL, Marfin AA. 2008. Increased recognition of Powassan encephalitis in the United States, 1999-2005. Vector Borne Zoonotic Dis 8:733–740. doi:10.1089/vbz.2008.0022. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

