Phosphatidylinositol-(4,5)-Bisphosphate Acyl Chains Differentiate Membrane Binding of HIV-1 Gag from That of the Phospholipase Cδ1 Pleckstrin Homology Domain
- PMID: 25995263
- PMCID: PMC4505672
- DOI: 10.1128/JVI.00794-15
Phosphatidylinositol-(4,5)-Bisphosphate Acyl Chains Differentiate Membrane Binding of HIV-1 Gag from That of the Phospholipase Cδ1 Pleckstrin Homology Domain
Abstract
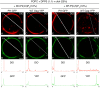
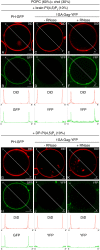
HIV-1 Gag, which drives virion assembly, interacts with a plasma membrane (PM)-specific phosphoinositide, phosphatidylinositol-(4,5)-bisphosphate [PI(4,5)P2]. While cellular acidic phospholipid-binding proteins/domains, such as the PI(4,5)P2-specific pleckstrin homology domain of phospholipase Cδ1 (PHPLCδ1), mediate headgroup-specific interactions with corresponding phospholipids, the exact nature of the Gag-PI(4,5)P2 interaction remains undetermined. In this study, we used giant unilamellar vesicles (GUVs) to examine how PI(4,5)P2 with unsaturated or saturated acyl chains affect membrane binding of PHPLCδ1 and Gag. Both unsaturated dioleoyl-PI(4,5)P2 [DO-PI(4,5)P2] and saturated dipalmitoyl-PI(4,5)P2 [DP-PI(4,5)P2] successfully recruited PHPLCδ1 to membranes of single-phase GUVs. In contrast, DO-PI(4,5)P2 but not DP-PI(4,5)P2 recruited Gag to GUVs, indicating that PI(4,5)P2 acyl chains contribute to stable membrane binding of Gag. GUVs containing PI(4,5)P2, cholesterol, and dipalmitoyl phosphatidylserine separated into two coexisting phases: one was a liquid phase, and the other appeared to be a phosphatidylserine-enriched gel phase. In these vesicles, the liquid phase recruited PHPLCδ1 regardless of PI(4,5)P2 acyl chains. Likewise, Gag bound to the liquid phase when PI(4,5)P2 had DO-acyl chains. DP-PI(4,5)P2-containing GUVs showed no detectable Gag binding to the liquid phase. Unexpectedly, however, DP-PI(4,5)P2 still promoted recruitment of Gag, but not PHPLCδ1, to the dipalmitoyl-phosphatidylserine-enriched gel phase of these GUVs. Altogether, these results revealed different roles for PI(4,5)P2 acyl chains in membrane binding of two PI(4,5)P2-binding proteins, Gag and PHPLCδ1. Notably, we observed that nonmyristylated Gag retains the preference for PI(4,5)P2 containing an unsaturated acyl chain over DP-PI(4,5)P2, suggesting that Gag sensitivity to PI(4,5)P2 acyl chain saturation is determined directly by the matrix-PI(4,5)P2 interaction, rather than indirectly by a myristate-dependent mechanism.
Importance: Binding of HIV-1 Gag to the plasma membrane is promoted by its interaction with a plasma membrane-localized phospholipid, PI(4,5)P2. Many cellular proteins are also recruited to the plasma membrane via PI(4,5)P2-interacting domains represented by PHPLCδ1. However, differences and/or similarities between these host proteins and viral Gag protein in the nature of their PI(4,5)P2 interactions, especially in the context of membrane binding, remain to be determined. Using a novel giant unilamellar vesicle-based system, we found that PI(4,5)P2 with an unsaturated acyl chain recruited PHPLCδ1 and Gag similarly, whereas PI(4,5)P2 with saturated acyl chains either recruited PHPLCδ1 but not Gag or sorted these proteins to different phases of vesicles. To our knowledge, this is the first study to show that PI(4,5)P2 acyl chains differentially modulate membrane binding of PI(4,5)P2-binding proteins. Since Gag membrane binding is essential for progeny virion production, the PI(4,5)P2 acyl chain property may serve as a potential target for anti-HIV therapeutic strategies.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

