A hard-wired priority map in the superior colliculus shaped by asymmetric inhibitory circuitry
- PMID: 25995346
- PMCID: PMC4512250
- DOI: 10.1152/jn.00144.2015
A hard-wired priority map in the superior colliculus shaped by asymmetric inhibitory circuitry
Abstract
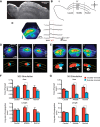
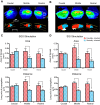
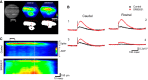
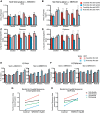
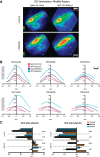
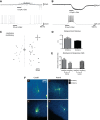
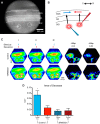
The mammalian superior colliculus (SC) is a laminar midbrain structure that translates visual signals into commands to shift the focus of attention and gaze. The SC plays an integral role in selecting targets and ultimately generating rapid eye movements to those targets. In all mammals studied to date, neurons in the SC are arranged topographically such that the location of visual stimuli and the endpoints of orienting movements form organized maps in superficial and deeper layers, respectively. The organization of these maps is thought to underlie attentional priority by assessing which regions of the visual field contain behaviorally relevant information. Using voltage imaging and patch-clamp recordings in parasagittal SC slices from the rat, we found the synaptic circuitry of the visuosensory map in the SC imposes a strong bias. Voltage imaging of responses to electrical stimulation revealed more spread in the caudal direction than the rostral direction. Pharmacological experiments demonstrated that this asymmetry arises from GABAA receptor activation rostral to the site of stimulation. Patch-clamp recordings confirmed this rostrally directed inhibitory circuit and showed that it is contained within the visuosensory layers of the SC. Stimulation of two sites showed that initial stimulation of a caudal site can take priority over subsequent stimulation of a rostral site. Taken together, our data indicate that the circuitry of the visuosensory SC is hard-wired to give higher priority to more peripheral targets, and this property is conferred by a uniquely structured, dedicated inhibitory circuit.
Keywords: electrophysiology; eye movements; motor layers; rat; rodent brain slice; superficial layers; vision; visual spatial attention; voltage imaging.
Copyright © 2015 the American Physiological Society.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

