Cell type- and activity-dependent extracellular correlates of intracellular spiking
- PMID: 25995352
- PMCID: PMC4509390
- DOI: 10.1152/jn.00628.2014
Cell type- and activity-dependent extracellular correlates of intracellular spiking
Abstract
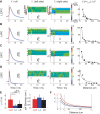
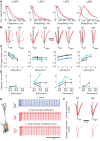
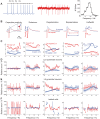
Despite decades of extracellular action potential (EAP) recordings monitoring brain activity, the biophysical origin and inherent variability of these signals remain enigmatic. We performed whole cell patch recordings of excitatory and inhibitory neurons in rat somatosensory cortex slice while positioning a silicon probe in their vicinity to concurrently record intra- and extracellular voltages for spike frequencies under 20 Hz. We characterize biophysical events and properties (intracellular spiking, extracellular resistivity, temporal jitter, etc.) related to EAP recordings at the single-neuron level in a layer-specific manner. Notably, EAP amplitude was found to decay as the inverse of distance between the soma and the recording electrode with similar (but not identical) resistivity across layers. Furthermore, we assessed a number of EAP features and their variability with spike activity: amplitude (but not temporal) features varied substantially (∼ 30-50% compared with mean) and nonmonotonically as a function of spike frequency and spike order. Such EAP variation only partly reflects intracellular somatic spike variability and points to the plethora of processes contributing to the EAP. Also, we show that the shape of the EAP waveform is qualitatively similar to the negative of the temporal derivative to the intracellular somatic voltage, as expected from theory. Finally, we tested to what extent EAPs can impact the lowpass-filtered part of extracellular recordings, the local field potential (LFP), typically associated with synaptic activity. We found that spiking of excitatory neurons can significantly impact the LFP at frequencies as low as 20 Hz. Our results question the common assertion that the LFP acts as proxy for synaptic activity.
Keywords: LFP; clustering; extracellular recordings; intracellular spikes; spike waveform.
Copyright © 2015 the American Physiological Society.
Figures




References
-
- Anastassiou CA, Koch C. Ephaptic coupling to endogenous electric field activity: why bother? Curr Opin Neurobiol 31: 95–103, 2015. - PubMed
-
- Anastassiou CA, Perin R, Markram H, Koch C. Ephaptic coupling of cortical neurons. Nat Neurosci 14: 217–223, 2011. - PubMed
-
- Anastassiou C, Buzsáki G, Koch C. Biophysics of extracellular spikes. In: Principles of Neural Coding. Boca Raton, FL: CRC Taylor, 2013, p. 15–37.
-
- Barthó P, Hirase H, Monconduit L, Zugaro M, Harris K, Buzsáki G. Characterization of neocortical principal cells and interneurons by network interactions and extracellular features. J Neurophysiol 92: 600–608, 2004. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

