Endosomal regulation of contact inhibition through the AMOT:YAP pathway
- PMID: 25995376
- PMCID: PMC4501364
- DOI: 10.1091/mbc.E15-04-0224
Endosomal regulation of contact inhibition through the AMOT:YAP pathway
Abstract
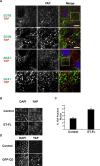
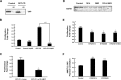
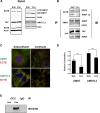
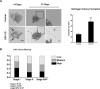
Contact-mediated inhibition of cell proliferation is an essential part of organ growth control; the transcription coactivator Yes-associated protein (YAP) plays a pivotal role in this process. In addition to phosphorylation-dependent regulation of YAP, the integral membrane protein angiomotin (AMOT) and AMOT family members control YAP through direct binding. Here we report that regulation of YAP activity occurs at the endosomal membrane through a dynamic interaction of AMOT with an endosomal integral membrane protein, endotubin (EDTB). EDTB interacts with both AMOT and occludin and preferentially associates with occludin in confluent cells but with AMOT family members in subconfluent cells. EDTB competes with YAP for binding to AMOT proteins in subconfluent cells. Overexpression of the cytoplasmic domain or full-length EDTB induces translocation of YAP to the nucleus, an overgrowth phenotype, and growth in soft agar. This increase in proliferation is dependent upon YAP activity and is complemented by overexpression of p130-AMOT. Furthermore, overexpression of EDTB inhibits the AMOT:YAP interaction. EDTB and AMOT have a greater association in subconfluent cells compared with confluent cells, and this association is regulated at the endosomal membrane. These data provide a link between the trafficking of tight junction proteins through endosomes and contact-inhibition-regulated cell growth.
© 2015 Cox et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures


References
-
- Basu S, Totty NF, Irwin MS, Sudol M, Downward J. Akt phosphorylates the Yes-associated protein, YAP, to induce interaction with 14-3-3 and attenuation of p73-mediated apoptosis. Mol Cell. 2003;11:11–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

