Basolateral amygdala response to food cues in the absence of hunger is associated with weight gain susceptibility
- PMID: 25995480
- PMCID: PMC4438134
- DOI: 10.1523/JNEUROSCI.3884-14.2015
Basolateral amygdala response to food cues in the absence of hunger is associated with weight gain susceptibility
Abstract
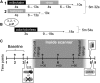
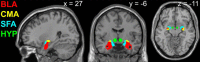
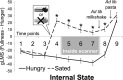
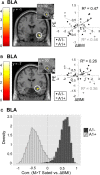
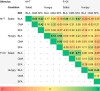
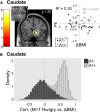
In rodents, food-predictive cues elicit eating in the absence of hunger (Weingarten, 1983). This behavior is disrupted by the disconnection of amygdala pathways to the lateral hypothalamus (Petrovich et al., 2002). Whether this circuit contributes to long-term weight gain is unknown. Using fMRI in 32 healthy individuals, we demonstrate here that the amygdala response to the taste of a milkshake when sated but not hungry positively predicts weight change. This effect is independent of sex, initial BMI, and total circulating ghrelin levels, but it is only present in individuals who do not carry a copy of the A1 allele of the Taq1A polymorphism. In contrast, A1 allele carriers, who have decreased D2 receptor density (Blum et al., 1996), show a positive association between caudate response and weight change. Regardless of genotype, however, dynamic causal modeling supports unidirectional gustatory input from basolateral amygdala (BLA) to hypothalamus in sated subjects. This finding suggests that, as in rodents, external cues gain access to the homeostatic control circuits of the human hypothalamus via the amygdala. In contrast, during hunger, gustatory inputs enter the hypothalamus and drive bidirectional connectivity with the amygdala. These findings implicate the BLA-hypothalamic circuit in long-term weight change related to nonhomeostatic eating and provide compelling evidence that distinct brain mechanisms confer susceptibility to weight gain depending upon individual differences in dopamine signaling.
Keywords: TaqIA; fMRI; feeding; metabolism; obesity; satiety.
Copyright © 2015 the authors 0270-6474/15/357964-13$15.00/0.
Figures



References
-
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, Habel U, Schneider F, Zilles K. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat Embryol (Berl) 2005;210:343–352. doi: 10.1007/s00429-005-0025-5. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
