Crystal structure of poly[μ2-aqua-aqua-(μ2-4-nitro-2,5,6-trioxo-1,2,5,6-tetra-hydro-pyridin-3-olato)hemi-μ4-oxalato-barium(II)]
- PMID: 25995855
- PMCID: PMC4420117
- DOI: 10.1107/S2056989015006520
Crystal structure of poly[μ2-aqua-aqua-(μ2-4-nitro-2,5,6-trioxo-1,2,5,6-tetra-hydro-pyridin-3-olato)hemi-μ4-oxalato-barium(II)]
Abstract
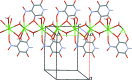
In the title coordination polymer, [Ba(C5HN2O6)(C2O4)0.5(H2O)2] n , the tenfold coordination of the Ba centre consists of four O atoms from the two 4-nitro-2,5,6-trioxo-1,2,5,6-tetra-hydro-pyridin-3-olate (L) anions, three O atoms of two oxalate anions and three water mol-ecules. The Ba-O bond lengths fall in the range 2.698 (3)-2.978 (3) Å. The L ligand chelates two Ba atoms related by a screw axis, leading to formation of fused five- and six-membered chelate rings. Due to the bridging function of the ligands and water mol-ecules, the complex monomers are connected into polymeric two-dimensional layers parallel to the bc plane. Inter-molecular O-H⋯O hydrogen bonds link these layers into a three-dimensional supra-molecular framework.
Keywords: barium(II) compound; crystal structure; oxalate; two-dimensional coordination polymer.
Figures
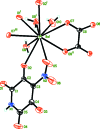
 , −z +
, −z +  ; (ii) −x, −y + 1, −z + 1; (iii) −x, y −
; (ii) −x, −y + 1, −z + 1; (iii) −x, y −  , −z +
, −z +  .]
.]

References
-
- Aldoshin, S. M. (2008). Russ. Chem. Bull. 57, 718–735.
-
- Belombe, M. M., Nenwa, J., Mbiangue, Y.-A., Nnanga, G. E., Mbomekalle, I.-M., Hey-Hawkins, E., Lonnecke, P. & Majoumo, F. (2003). Dalton Trans. pp. 2117–2118.
-
- Belombe, M. M., Nenwa, J., Tchuisse, N. A. S. N., Emmerling, F., Obbade, S., Semmoud, A. & Fokwa, B. P. T. (2012). ScienceJet, 1, 24–27.
-
- Bouayad, A., Trombe, J.-C. & Gleizes, A. (1995). Inorg. Chim. Acta, 230, 1–7.
-
- Bruker (2004). APEX2, SAINT and SADABS. Bruker AXS Inc., Madison, Wisconsin, USA.
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
