Chemistry and biological activity of ramalina lichenized fungi
- PMID: 25996207
- PMCID: PMC6272487
- DOI: 10.3390/molecules20058952
Chemistry and biological activity of ramalina lichenized fungi
Abstract
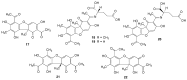
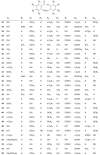
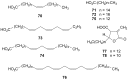
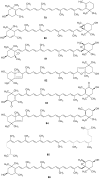
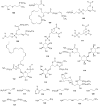
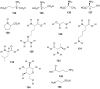
Lichens are a form of symbiont between a fungus and an alga or cyanobacterium, which contains a wide variety of organic compounds with certain secondary metabolite classes typical of these organisms. The Ramalina genus has approximately 246 species distributed around the World, of which in this review approximately 118 species with published chemical or biological activity studies of extracts or isolated compounds were cited. From the 153 mentioned compounds, only 27 passed were tested for biological activity, being usnic acid the most studied compound and the one showing the best results in almost all in vitro tests performed, although other compounds also presented excellent results as antimicrobial, antitumor and anti-inflammatory agents, among others. Extracts of several species also presented significant results in performed biological tests, demonstrating the potential that these organisms have, in particular, the gender Ramalina, to produce bioactive molecules that can be used as a model for the production of pharmaceuticals.
Keywords: Ramalina; antitumoral; biological activitiy; lichen; usnic acid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Huneck S., Yoshimura I. Identification of Lichen Substances. Springer-Verlag; Berlin, Germany: 1996.
-
- Boustie J., Grube M. Lichens—A promising source of bioactive secondary metabolites. Plant Genet. Resour. 2005;3:273–287. doi: 10.1079/PGR200572. - DOI
-
- Honda N.K., Vilegas W. A química dos liquens. Quim. Nova. 1998;21:110–125.
-
- Feige G.B., Lumbsch H.T. Identification of lichen substances by a standardized high-performance liquid chromatographic method. J. Chromatogr. 1993;647:417–427. doi: 10.1016/0021-9673(93)83356-W. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

