Darbepoetin administration to neonates undergoing cooling for encephalopathy: a safety and pharmacokinetic trial
- PMID: 25996892
- PMCID: PMC5564328
- DOI: 10.1038/pr.2015.101
Darbepoetin administration to neonates undergoing cooling for encephalopathy: a safety and pharmacokinetic trial
Abstract
Background: Despite therapeutic hypothermia, neonates with encephalopathy (NE) have high rates of death or disability. Darbepoetin alfa (Darbe) has comparable biological activity to erythropoietin, but has extended circulating half-life (t(1/2)). Our aim was to determine Darbe safety and pharmacokinetics as adjunctive therapy to hypothermia.
Study design: Thirty infants (n = 10/arm) ≥36 wk gestation undergoing therapeutic hypothermia for NE were randomized to receive placebo, Darbe low dose (2 μg/kg), or high dose (10 μg/kg) given intravenously within 12 h of birth (first dose/hypothermia condition) and at 7 d (second dose/normothermia condition). Adverse events were documented for 1 mo. Serum samples were obtained to characterize Darbe pharmacokinetics.
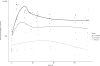
Results: Adverse events (hypotension, altered liver and renal function, seizures, and death) were similar to placebo and historical controls. Following the first Darbe dose at 2 and 10 μg/kg, t(1/2) was 24 and 32 h, and the area under the curve (AUC(inf)) was 26,555 and 180,886 h*mU/ml*, respectively. In addition, clearance was not significantly different between the doses (0.05 and 0.04 l/h). At 7 d, t(1/2) was 26 and 35 h, and AUC(inf) was 10,790 and 56,233 h*mU/ml*, respectively (*P < 0.01).
Conclusion: Darbe combined with hypothermia has similar safety profile to placebo with pharmacokinetics sufficient for weekly administration.
Conflict of interest statement
Disclosures: M.C.B. and the coauthors have no financial ties to products in the study and no potential/perceived conflict of interest.
Figures
References
-
- Graham EM, Ruis KA, Hartman AL, Northington FJ, Fox HE. A systematic review of the role of intrapartum hypoxia-ischemia in the causation of neonatal encephalopathy. Am J Obstet Gynecol. 2008;199:587–95. - PubMed
-
- Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2014;385:430–40. - PubMed
-
- Shankaran S, Woldt E, Koepke T, Bedard MP, Nandyal R. Acute neonatal morbidity and long-term central nervous system sequelae of perinatal asphyxia in term infants. Early Hum Dev. 1991;25:135–48. - PubMed
-
- Robertson CM, Finer NN, Grace MG. School performance of survivors of neonatal encephalopathy associated with birth asphyxia at term. J Pediatr. 1989;114:753–60. - PubMed
-
- Shankaran S, Laptook AR, Ehrenkranz RA, et al. National Institute of Child Health and Human Development Neonatal Research Network Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials