Gammaherpesvirus Co-infection with Malaria Suppresses Anti-parasitic Humoral Immunity
- PMID: 25996913
- PMCID: PMC4440701
- DOI: 10.1371/journal.ppat.1004858
Gammaherpesvirus Co-infection with Malaria Suppresses Anti-parasitic Humoral Immunity
Abstract
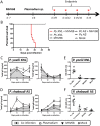
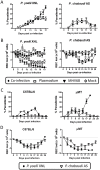
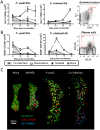
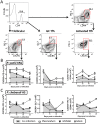
Immunity to non-cerebral severe malaria is estimated to occur within 1-2 infections in areas of endemic transmission for Plasmodium falciparum. Yet, nearly 20% of infected children die annually as a result of severe malaria. Multiple risk factors are postulated to exacerbate malarial disease, one being co-infections with other pathogens. Children living in Sub-Saharan Africa are seropositive for Epstein Barr Virus (EBV) by the age of 6 months. This timing overlaps with the waning of protective maternal antibodies and susceptibility to primary Plasmodium infection. However, the impact of acute EBV infection on the generation of anti-malarial immunity is unknown. Using well established mouse models of infection, we show here that acute, but not latent murine gammaherpesvirus 68 (MHV68) infection suppresses the anti-malarial humoral response to a secondary malaria infection. Importantly, this resulted in the transformation of a non-lethal P. yoelii XNL infection into a lethal one; an outcome that is correlated with a defect in the maintenance of germinal center B cells and T follicular helper (Tfh) cells in the spleen. Furthermore, we have identified the MHV68 M2 protein as an important virus encoded protein that can: (i) suppress anti-MHV68 humoral responses during acute MHV68 infection; and (ii) plays a critical role in the observed suppression of anti-malarial humoral responses in the setting of co-infection. Notably, co-infection with an M2-null mutant MHV68 eliminates lethality of P. yoelii XNL. Collectively, our data demonstrates that an acute gammaherpesvirus infection can negatively impact the development of an anti-malarial immune response. This suggests that acute infection with EBV should be investigated as a risk factor for non-cerebral severe malaria in young children living in areas endemic for Plasmodium transmission.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Expansion of murine gammaherpesvirus latently infected B cells requires T follicular help.PLoS Pathog. 2014 May 1;10(5):e1004106. doi: 10.1371/journal.ppat.1004106. eCollection 2014 May. PLoS Pathog. 2014. PMID: 24789087 Free PMC article.
-
T Cell-Intrinsic Interferon Regulatory Factor 1 Expression Suppresses Differentiation of CD4+ T Cell Populations That Support Chronic Gammaherpesvirus Infection.J Virol. 2021 Sep 27;95(20):e0072621. doi: 10.1128/JVI.00726-21. Epub 2021 Aug 4. J Virol. 2021. PMID: 34346769 Free PMC article.
-
Murine gammaherpesvirus M2 antigen modulates splenic B cell activation and terminal differentiation in vivo.PLoS Pathog. 2017 Aug 2;13(8):e1006543. doi: 10.1371/journal.ppat.1006543. eCollection 2017 Aug. PLoS Pathog. 2017. PMID: 28767707 Free PMC article.
-
Does EBV alter the pathogenesis of malaria?Parasite Immunol. 2015 Sep;37(9):433-45. doi: 10.1111/pim.12212. Parasite Immunol. 2015. PMID: 26121587 Free PMC article. Review.
-
Murine Gammaherpesvirus 68: A Small Animal Model for Gammaherpesvirus-Associated Diseases.Adv Exp Med Biol. 2017;1018:225-236. doi: 10.1007/978-981-10-5765-6_14. Adv Exp Med Biol. 2017. PMID: 29052141 Review.
Cited by
-
IFNAR1-Signalling Obstructs ICOS-mediated Humoral Immunity during Non-lethal Blood-Stage Plasmodium Infection.PLoS Pathog. 2016 Nov 3;12(11):e1005999. doi: 10.1371/journal.ppat.1005999. eCollection 2016 Nov. PLoS Pathog. 2016. PMID: 27812214 Free PMC article.
-
To B or Not to B: Understanding B Cell Responses in the Development of Malaria Infection.Front Immunol. 2018 Dec 14;9:2961. doi: 10.3389/fimmu.2018.02961. eCollection 2018. Front Immunol. 2018. PMID: 30619319 Free PMC article. Review.
-
Conserved Gammaherpesvirus Protein Kinase Selectively Promotes Irrelevant B Cell Responses.J Virol. 2019 Apr 3;93(8):e01760-18. doi: 10.1128/JVI.01760-18. Print 2019 Apr 15. J Virol. 2019. PMID: 30728267 Free PMC article.
-
Follicular Helper T Cells are Essential for the Elimination of Plasmodium Infection.EBioMedicine. 2017 Oct;24:216-230. doi: 10.1016/j.ebiom.2017.08.030. Epub 2017 Sep 4. EBioMedicine. 2017. PMID: 28888925 Free PMC article.
-
B-cell memory in malaria: Myths and realities.Immunol Rev. 2020 Jan;293(1):57-69. doi: 10.1111/imr.12822. Epub 2019 Nov 16. Immunol Rev. 2020. PMID: 31733075 Free PMC article. Review.
References
-
- World Health Organization. Global Malaria Programme. (2012) World malaria report 2012 Geneva: World Health Organization; xxxiv, 249 p. p.
-
- Gupta S, Snow RW, Donnelly CA, Marsh K, Newbold C (1999) Immunity to non-cerebral severe malaria is acquired after one or two infections. Nat Med 5: 340–343. - PubMed
-
- Miller LH, Baruch DI, Marsh K, Doumbo OK (2002) The pathogenic basis of malaria. Nature 415: 673–679. - PubMed
-
- Greenwood B, Marsh K, Snow R (1991) Why do some African children develop severe malaria? Parasitol Today 7: 277–281. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

