Role of hypoxia-inducible factor-1α and CD146 in epidermal growth factor receptor-mediated angiogenesis in salivary gland adenoid cystic carcinoma
- PMID: 25997612
- PMCID: PMC4526044
- DOI: 10.3892/mmr.2015.3815
Role of hypoxia-inducible factor-1α and CD146 in epidermal growth factor receptor-mediated angiogenesis in salivary gland adenoid cystic carcinoma
Erratum in
-
[Corrigendum] Role of hypoxia‑inducible factor‑1α and CD146 in epidermal growth factor receptor‑mediated angiogenesis in salivary gland adenoid cystic carcinoma.Mol Med Rep. 2026 Mar;33(3):91. doi: 10.3892/mmr.2026.13801. Epub 2026 Jan 16. Mol Med Rep. 2026. PMID: 41543191 Free PMC article.
Abstract
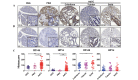
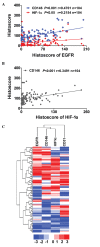
Adenoid cystic carcinoma (AdCC) of the salivary gland in the head and neck is characterized by indolent yet persistent growth, multiple local recurrences and early hematogenous metastasis. Considering the possible association between the epidermal growth factor receptor (EGFR) signaling pathway and angiogenesis in various types of cancer and the overexpression of EGFR in AdCC, it is reasonable to examine the correlation between angiogenesis and the EGFR signaling pathway in this carcinoma. In the present study, the expression of EGFR, CD31, CD146 and hypoxia‑inducible factor‑1α (HIF‑1α) were evaluated by immunohistochemical staining with tissue microarray containing normal salivary gland (NSG), pleomorphic adenoma (PMA) and AdCC tissues. Pearson's correlation coefficient was conducted to demonstrate the correlation between EGFR, CD31, CD146 and HIF‑1α. To determine their similarity and intimacy, hierarchical analysis was performed with Cluster 3.0 and then visualized using TreeView software. Immunohistochemical results of tissue microarrays were quantified, revealing that the expression of EGFR, CD146 and HIF‑1α increased in AdCC compared with in PMA and NSG tissues. The association between the expression of EGFR and CD31 was significant and positive. The expression of CD146 and HIF‑1α was positively correlated with EGFR and CD31, respectively. These findings suggest that the EGFR signaling pathway has a vital role in AdCC progression and may be associated with HIF‑1α‑mediated angiogenesis. These results may enhance our understanding of the mechanism underlying AdCC progression and provide potential clinical therapeutic strategies based on the inhibition of EGFR.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

