Cost-effectiveness of abatacept, rituximab, and TNFi treatment after previous failure with TNFi treatment in rheumatoid arthritis: a pragmatic multi-centre randomised trial
- PMID: 25997746
- PMCID: PMC4489004
- DOI: 10.1186/s13075-015-0630-5
Cost-effectiveness of abatacept, rituximab, and TNFi treatment after previous failure with TNFi treatment in rheumatoid arthritis: a pragmatic multi-centre randomised trial
Abstract
Introduction: For patients with rheumatoid arthritis (RA) whose treatment with a tumour necrosis factor inhibitor (TNFi) is failing, several biological treatment options are available. Often, another TNFi or a biological with another mode of action is prescribed. The objective of this study was to compare the effectiveness and cost-effectiveness of three biologic treatments with different modes of action in patients with RA whose TNFi therapy is failing.
Methods: We conducted a pragmatic, 1-year randomised trial in a multicentre setting. Patients with active RA despite previous TNFi treatment were randomised to receive abatacept, rituximab or a different TNFi. The primary outcome (Disease Activity Score in 28 joints) and the secondary outcomes (Health Assessment Questionnaire Disability Index and 36-item Short Form Health Survey scores) were analysed using linear mixed models. Cost-effectiveness was analysed on the basis of incremental net monetary benefit, which was based on quality-adjusted life-years (calculated using EQ-5D scores), and all medication expenditures consumed in 1 year. All analyses were also corrected for possible confounders.
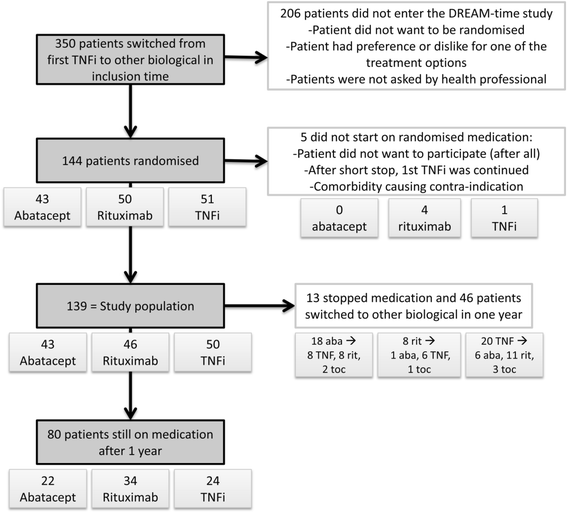
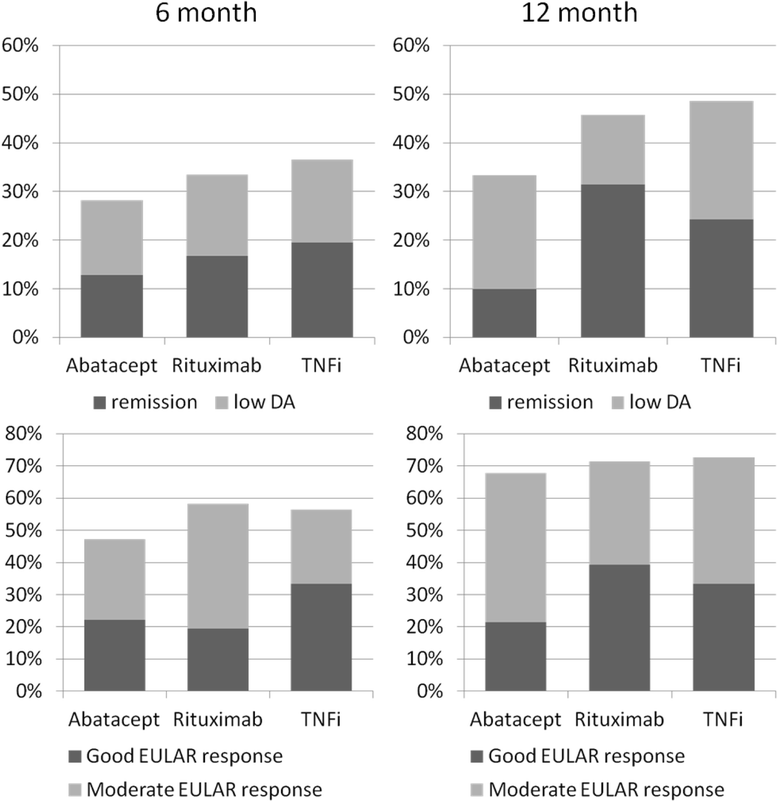
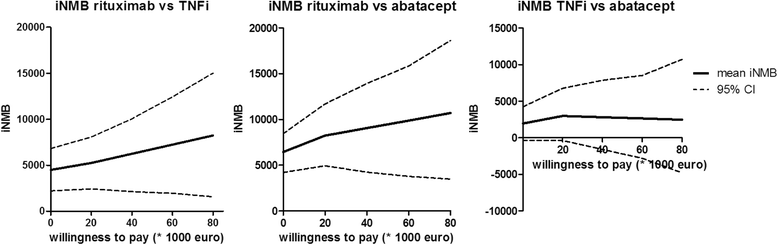
Results: Of 144 randomised patients, 5 were excluded and 139 started taking abatacept (43 patients), rituximab (46 patients) or a different TNFi (50 patients). There were no significant differences between the three groups with respect to multiple measures of RA outcomes. However, our analysis revealed that rituximab therapy is significantly more cost-effective than both abatacept and TNFi over a willingness-to-pay range of 0 to 80,000 euros.
Conclusions: All three treatment options were similarly effective; however, when costs were factored into the treatment decision, rituximab was the best option available to patients whose first TNFi treatment failed. However, generalization of these costs to other countries should be undertaken carefully.
Trial registration: Netherlands Trial Register number NTR1605. Registered 24 December 2008.
Figures


References
-
- Jobanputra P, Maggs F, Deeming A, Carruthers D, Rankin E, Jordan AC, et al. A randomised efficacy and discontinuation study of etanercept versus adalimumab (RED SEA) for rheumatoid arthritis: a pragmatic, unblinded, non-inferiority study of first TNF inhibitor use: outcomes over 2 years. BMJ Open. 2012;2:e001395. doi: 10.1136/bmjopen-2012-001395. - DOI - PMC - PubMed
-
- Hyrich KL, Watson KD, Lunt M, Symmons DPM, British Society for Rheumatology Biologics Register (BSRBR) Changes in disease characteristics and response rates among patients in the United Kingdom starting anti-tumour necrosis factor therapy for rheumatoid arthritis between 2001 and 2008. Rheumatology. 2011;50:117–123. doi: 10.1093/rheumatology/keq209. - DOI - PMC - PubMed
-
- Haraoui B, Keystone EC, Thorne JC, Pope JE, Chen I, Asare CG, et al. Clinical outcomes of patients with rheumatoid arthritis after switching from infliximab to etanercept. J Rheumatol. 2004;31:2356–2359. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

