Building better monoclonal antibody-based therapeutics
- PMID: 25998715
- PMCID: PMC4491443
- DOI: 10.1038/nrc3930
Building better monoclonal antibody-based therapeutics
Abstract
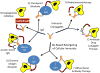
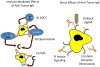
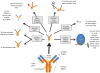
For 20 years, monoclonal antibodies (mAbs) have been a standard component of cancer therapy, but there is still much room for improvement. Efforts continue to build better cancer therapeutics based on mAbs. Anticancer mAbs function through various mechanisms, including directly targeting the malignant cells, modifying the host response, delivering cytotoxic moieties and retargeting cellular immunity towards the malignant cells. Characteristics of mAbs that affect their efficacy include antigen specificity, overall structure, affinity for the target antigen and how a mAb component is incorporated into a construct that can trigger target cell death. This Review discusses the various approaches to using mAb-based therapeutics to treat cancer and the strategies used to take advantage of the unique potential of each approach, and provides examples of current mAb-based treatments.
Figures
References
-
- Ehrlich P. Collected studies on immunity. 1. J. Wiley & sons; 1906.
-
- Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495. Landmark publication outlining both how to produce monoclonal antibodies as well as their therapeutic potential. - PubMed
-
- Milstein C. From the structure of antibodies to the diversification of the immune response. Nobel lecture, 8 December 1984. Bioscience reports. 1985;5:275–297. - PubMed
-
- Bernard A, Boumsell L. The clusters of differentiation (CD) defined by the First International Workshop on Human Leucocyte Differentiation Antigens. Hum Immunol. 1984;11:1–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

