Type 2 diabetes as a protein misfolding disease
- PMID: 25998900
- PMCID: PMC4492843
- DOI: 10.1016/j.molmed.2015.04.005
Type 2 diabetes as a protein misfolding disease
Abstract
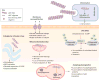
Type 2 diabetes (T2D) is a highly prevalent and chronic metabolic disorder. Recent evidence suggests that formation of toxic aggregates of the islet amyloid polypeptide (IAPP) might contribute to β-cell dysfunction and disease. However, the mechanism of protein aggregation and associated toxicity remains unclear. Misfolding, aggregation, and accumulation of diverse proteins in various organs is the hallmark of the group of protein misfolding disorders (PMDs), including highly prevalent illnesses affecting the central nervous system (CNS) such as Alzheimer's disease (AD) and Parkinson's disease (PD). In this review we discuss the current understanding of the mechanisms implicated in the formation of protein aggregates in the endocrine pancreas and associated toxicity in the light of the long-standing knowledge from neurodegenerative disorders associated with protein misfolding.
Keywords: amyloid; islet amyloid polypeptide; prions; protein misfolding; type 2 diabetes; β cells.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures

References
-
- Soto C. Unfolding the role of protein misfolding in neurodegenerative diseases. Nat Rev Neurosci. 2003;4:49–60. - PubMed
-
- Hardy J, Gwinn-Hardy K. Genetic classification of primary neurodegenerative disease. Science. 1998;282:1075–1079. - PubMed
-
- Moreno-Gonzalez I, Soto C. Natural animal models of neurodegenerative protein misfolding diseases. Curr Pharm Des. 2012;18:1148–1158. - PubMed
-
- Butler AE, et al. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

